250. J. Zhang, K.K. Cheunget al. Enantioconvergent Hydrobenzylation of Racemic Allylic Alcohols with Aryl Hydrazones via Bifunctional Ruthenium Catalysis. J. Am. Chem. Soc. 2025, 147, 27790.
249. M, Zhang, N. Zhanget al. Cobalt pincer complex-catalyzed highly enantioselective hydrogenation of quinoxalines. Chin. Chem. Lett. 2025, 36, 110081.
248. C, Wang and J. Xiao. Activation of Molecular Oxygen and Selective Oxidation with Metal Complexes. Acc. Chem. Res. 2025.
247. J. Xiao. Neither H2 nor O2 in hydrogenative oxidations with water. Nat. Catal. 2025, 8, 96-97.
246. Y. Zheng, Z. Chen, et al. Dynamic kinetic resolution-mediated synthesis of C-3 hydroxylated arginine derivatives. R. Soc. Open Sci. 2025, 12: 241607.
245. I. Calisir, E. Bennett, et al. Designing a filler material to reduce dielectric loss in epoxy-based substrates for high-frequency applications. RSC Adv. 2025, 15, 754-763.
244. L.Qi, Z. Chen, et al. Reductive Zincke Reaction: Opening of Pyridinium Rings to δ-Amino Ketones via Transfer Hydrogenation. Chem. Eur. J. 2025, e202404043.
243. R, Guan, T. Wang, et al. Cerium-Catalyzed Ritter-Type C−H Amination of Alkylarenes. Eur. J. Org. Chem. 2025, e202400935.
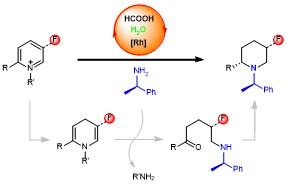
242. Z, Chen, G. Song, et al. Reductive Transamination of Pyridinium Salts to N-Aryl Piperidines. J. Org. Chem. 2024, 89, 9352-9359.
241. M. Zhang, N. Zhang, et al. Cobalt pincer complex-catalyzed highly enantioselective hydrogenation of quinoxalines. Chin. Chem. Lett. 2024, 110081
240. S. Williams, L. Qi, et al. Hydrogenation of functionalised pyridines with a rhodium oxide catalyst under mild conditions. Org. Biomol. Chem. 2024, 22, 1010-1017
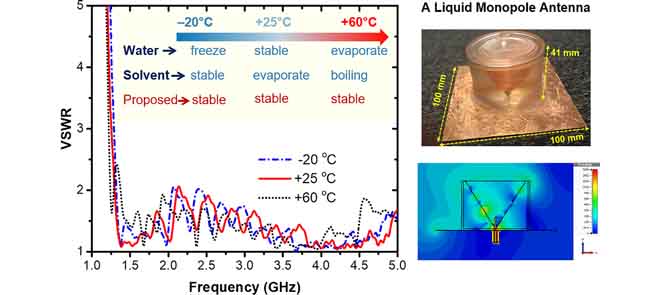
239. E. Bennett, I. Calisir, et al. Correlation of Dielectric Properties with Structure and H-Bonding for Liquids. J. Phys. Chem. C. 2023, 127, 18669-18677
238. P. Kumar, L. Qi, et al. Selective Hydrogenation of Lignin-derived Aromatics to Give Cyclohexanes with a Rhodium-Pincer Precatalyst. J. Organomet. Chem. 2023, 997, 122795
237. R. Guan, G. Chen, et al. Chemoselective Decarboxylative Oxygenation of Carboxylic Acids To Access Ketones, Aldehydes, and Peroxides. Org. Lett. 2023, 25, 2482-2486.
236. I. Calisir, X. Yang, et al. Enhancing the bandwidth of antennas using polymer composites with high dielectric relaxation. Mater. Today Commun. 2023, 100026.
235. J. Wu, Z. Chen, et al. Synthesis of chiral piperidines from pyridinium salts via rhodium-catalysed transfer hydrogenation. Nat. catal. 2022, 5, 982-992.
234. J. Zhou, M. Jia, et al. Chemoselective Oxyfunctionalization of Functionalized Benzylic Compounds with a Manganese Catalyst. Angew. Chem. Int. Ed. 2022, e202205983.
233. Z. Huang, R. Guan, et al. Non-heme manganese(ii) complex-catalysed oxidative cleavage of 1,2-diols via alcohol-assisted O2 activation. Green Chem 2022, 24, 3814-3823.
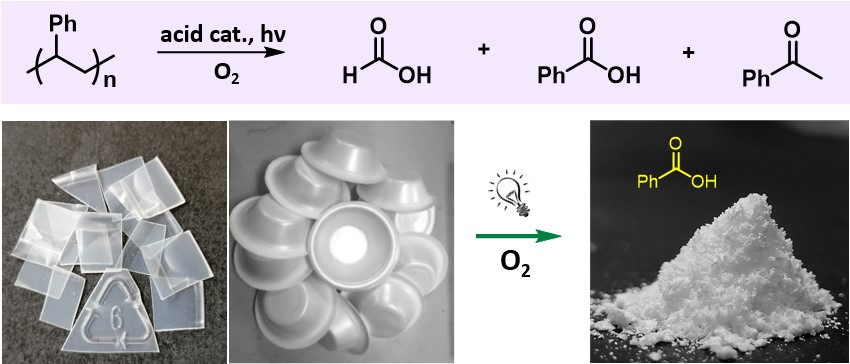
232. Z. Huang, M. Shanmugam, et al. Chemical Recycling of Polystyrene to Valuable Chemicals via Selective Acid-Catalyzed Aerobic Oxidation under Visible Light. J. Am. Chem. Soc. 2022, 144, 6532-6542.
231. R. Guan, E. Bennett, et al. Decarboxylative oxygenation of carboxylic acids with O2via a non-heme manganese catalyst. Green Chem 2022, 24, 2946-2952.
230. R. Gunasekar, R. Goodyear, et al. Recent developments in enantio- and diastereoselective hydrogenation of N-heteroaromatic compounds. Org. Biomol. Chem. 2022, 20, 1794-1827.
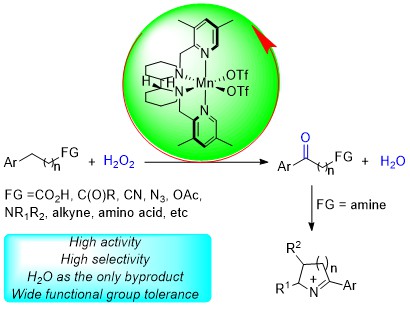
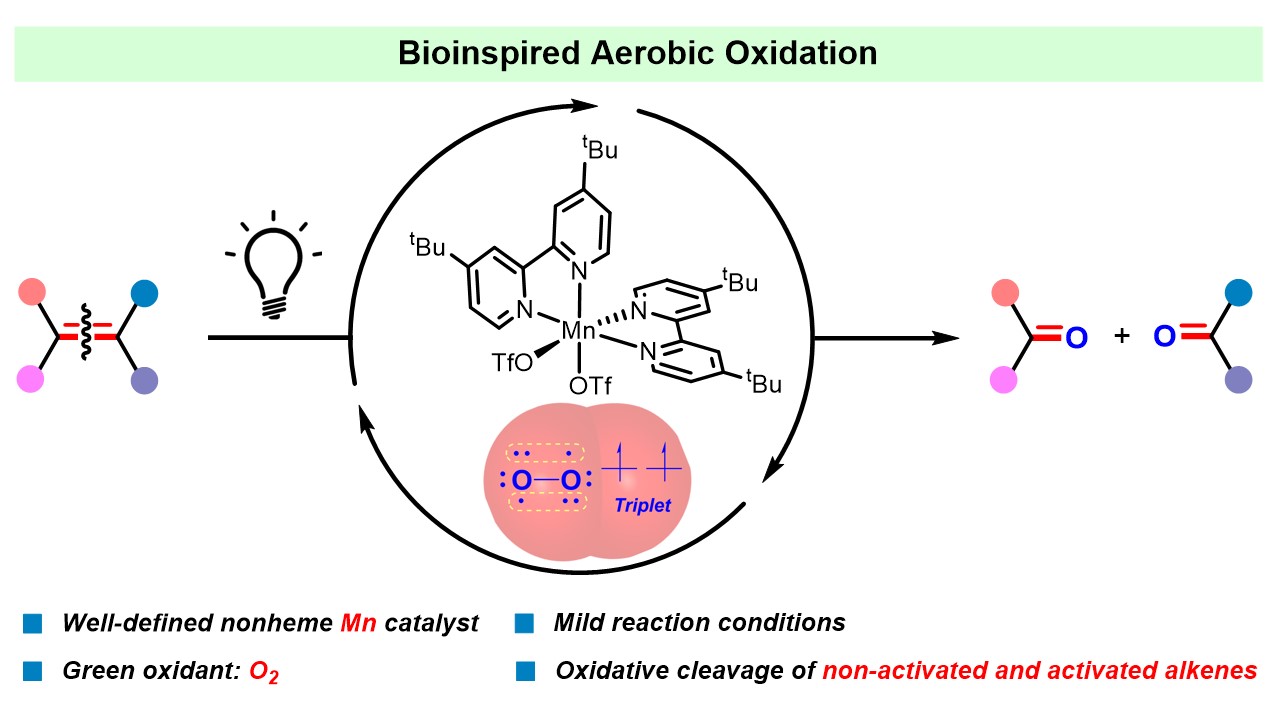
229. Z. Huang, R. Guan, et al. Oxidative Cleavage of Alkenes by O2 with a Non-Heme Manganese Catalyst. J. Am. Chem. Soc. 2021, 143, 10005-10013.
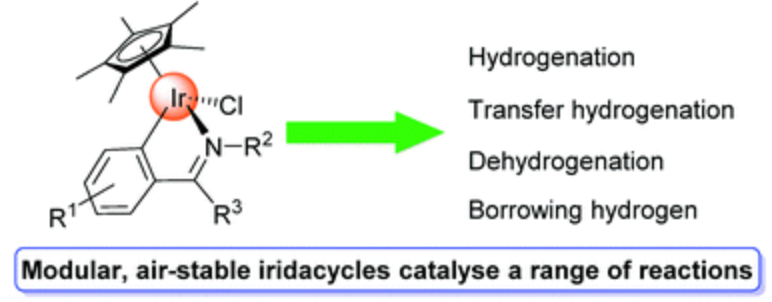
228. Z. Chen, A. Kacmaz, et al. Recent Development in the Synthesis and Catalytic Application of Iridacycles. Chem. Rec. 2021, 21, 1-30.
227. J. Smith, A. Kacmaz, et al. Chiral cyclometalated iridium complexes for asymmetric reduction reactions. Org. Biomol. Chem. 2021, 19, 279-284.
226. C. Song, E. Bennett, et al. Multi-Mode Hybrid Antennas Using Liquid Dielectric Resonator and Magneto-Electric Dipole. IEEE T. Antenn. Propag. 2020.
225. R. Xu, K. Wang, et al. Anti-Markovnikov Hydroamination of Racemic Allylic Alcohols to Access Chiral g-Amino Alcohols. Agew. Chem. Int. Ed. 2020, 59, 21959-21964.
224. Z. Chen, G. Chen, et al. Methanol as Hydrogen Source: Transfer Hydrogenation of Aldehydes near Room Temperature. Asian J. Org. Chem. 2020, 9, 1-6.
223. Y. Liu, X. Yue, et al. Asymmetric Induction with a Chiral Amine Catalyzed by a Ru-PNP Pincer Complex: Insight from Theoretical Investigation. Inorg. Chem. 2020, 59, 8404–8411.
222. L. Yang, H. Lu, et al. Light‐Promoted Nickel Catalysis: Etherification of Aryl Electrophiles with Alcohols Catalyzed by a NiII‐Aryl Complex. Angew. Chem. Int. Ed. 2020, 59, 12714 –12719.
221. K. Wang, L. Zhang, et al. Asymmetric Guerbet Reaction to Access Chiral Alcohols. Angew. Chem. Int. Ed. 2020, 59, 11408 –11415.
220. R. Miao, D. Wang, et al. Halogen Bonding Matters: Visible Light-Induced Photoredox Catalyst-Free Aryl Radical Formation and Its Applications. Phys. Chem. Chem. Phys. 2020, 22, 10212-10218.
219. Y. Liu, Y. Yan, et al. Highly Efficient Binuclear Copper‐Catalyzed Oxidation of N,N‐Dimethylanilines with O2. ChemCatChem 2020, 12, 2221 –2225.
218. T. Song, Z. Ma, et al. A Bifunctional Iron Nanocomposite Catalyst for Efficient Oxidation of Alkenes to Ketones and 1,2-Diketones. ACS Catal. 2020, 10, 4617–4629.
217. C. Song, E. L. Bennett, et al. Passive Beam-Steering Gravitational Liquid Antennas. IEEE Trans. Antennas Propag. 2020, 68, 3207-3212.
216. T. Song, P. Ren, et al. Highly Dispersed Ni2P Nanoparticles on N,P-Codoped Carbon for Efficient Cross-Dehydrogenative Coupling to Access Alkynyl Thioethers. Green Chem. 2020, 22, 651-656.
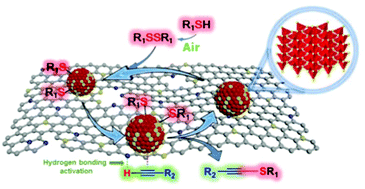
215. T. Song, P. Ren, et al. Highly Dispersed Single-Phase Ni2P Nanoparticles on N,P-Codoped Porous Carbon for Efficient Synthesis of N-Heterocycles. ACS Sustainable Chem. Eng. 2020, 8, 267–277.
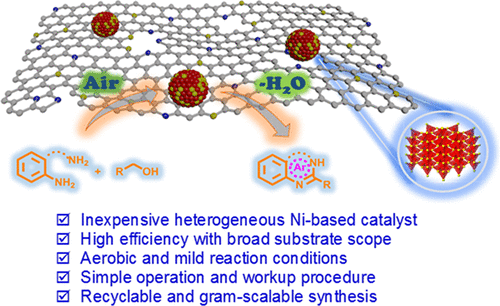
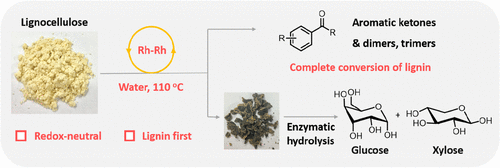
214. Y. Liu, C. Li, et al. Rhodium-Terpyridine Catalyzed Redox-Neutral Depolymerization of Lignin in Water. Green Chem. 2020, 22, 33-38.
213. E. L. Bennett, C. Song, et al. Measured Relative Complex Permittivities for Multiple Series of Ionic Liquids. J. Mol. Liq. 2019, 294, 111571.
212. Y. Duan, X. Dona, et al. Hydrogenation of Functionalized Nitroarenes Catalyzed by Single‐Phase Pyrite FeS2 Nanoparticles on N,S‐Codoped Porous Carbon. ChemSusChem 2019, 12, 4636 –4644.
211. R. Begum, Z. H. Farooqi, et al. Reduction of Nitroarenes Catalyzed by Microgel-Stabilized Silver Nanoparticles. J. Hazard. Mater. 2019, 377, 399–408.
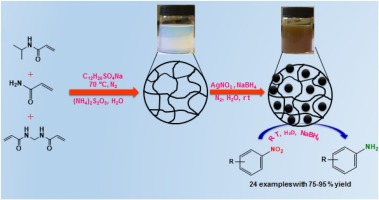
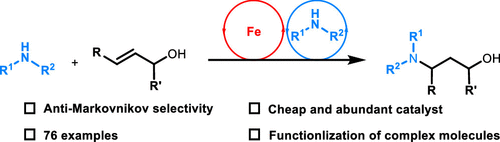
210. W. Ma, X. Zhang, et al. Iron-Catalyzed Anti-Markovnikov Hydroamination and Hydroamidation of Allylic Alcohols. J. Am. Chem. Soc. 2019, 141, 13506–13515.
209. M. Xiao, X. Yue, et al. Transition‐Metal‐Free Hydrogen Autotransfer: Diastereoselective N‐Alkylation of Amines with Racemic Alcohols. Angew. Chem. Int. Ed. 2019, 58, 10528 –10536.
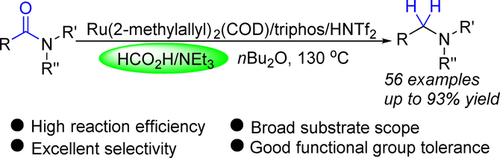
208. Y. Pan, Z. Luo, et al. Ru‐Catalyzed Deoxygenative Transfer Hydrogenation of Amides to Amines with Formic Acid/Triethylamine. Adv. Synth. Catal. 2019, 361, 3800-3806.
207. C. Song, E. L. Bennett, et al. Metasurfaced, Broadband, and Circularly Polarized Liquid Antennas Using a Simple Structure. IEEE Trans. Antennas Propag. 2019, 67, 4907-4913.
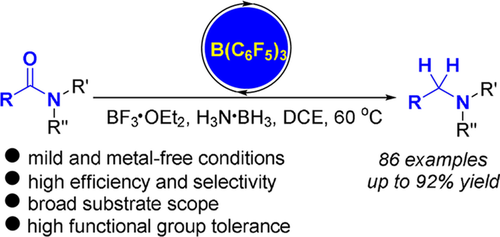
206. Y. Pan, Z. Luo, et al. B(C6F5)3‐Catalyzed Deoxygenative Reduction of Amides to Amines with Ammonia Borane. Adv. Synth. Catal. 2019, 361, 2301 –2308.
205. K. Wang, J. Zhou, et al. Selective Manganese‐Catalyzed Oxidation of Hydrosilanes to Silanols under Neutral Reaction Conditions. Angew. Chem. Int. Ed. 2019, 58, 6380 –6384.
204. Y. Liu, C. Li, et al. Mild Redox-Neutral Depolymerization of Lignin with a Binuclear Rh Complex in Water. ACS Catal. 2019, 9, 4441–4447.
203. A. Gonzalez‐de‐Castro, C. M. Robertson, et al. Boosting Molecular Complexity with O2: Iron‐Catalysed Oxygenation of 1‐Arylisochromans through Dehydrogenation, Csp3−O Bond Cleavage and Hydrogenolysis. Chem. Eur. J. 2019, 25, 4345 –4357.
202. J. Ruan, J. A.Iggo, et al. Effect of Hydrogen Bonding on Ligand Substitution and Its Implication for the Heck Reaction. J. Organometal. Chem. 2019, 880, 150-155.
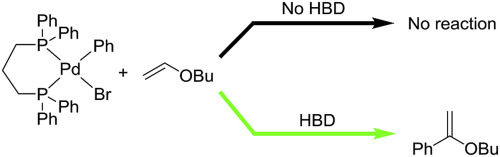
201. C. Song, E. L. Bennett, et al. Compact Ultra-Wideband Monopole Antennas Using Novel Liquid Loading Materials. IEEE Access. 2019, 7, 49039-49047.
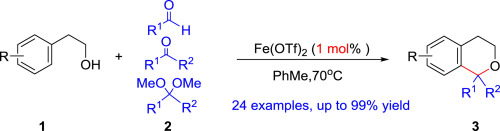
200. J. Zhou, C. Wang, et al. Synthesis of Isochromans via Fe(OTf)2-Catalyzed Oxa-Pictet–Spengler Cyclization. Tetrahedron. 2018, 74, 7040-7046.
199. J. Yang, S. Chen, et al. Cobalt-Catalyzed α-Methoxymethylation and Aminomethylation of Ketones with Methanol as a C1 Source. Org. Lett. 2018, 20, 6774–6779.
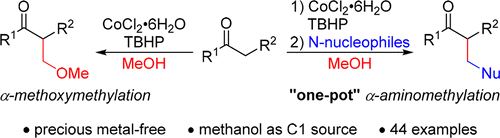
198. W. Zhou, Q. Tao, et al. Additive-Free Aerobic Oxidative Dehydrogenation of N-Heterocycles under Catalysis by NiMn Layered Hydroxide Compounds. J. Catal. 2018, 361, 1–11.
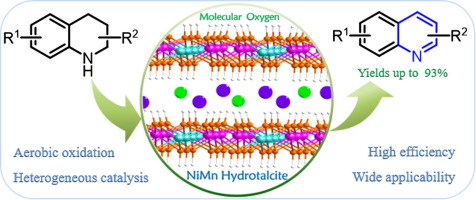
197. Y. Pan, C. Chen, et al. Metal-Free Tandem Cyclization/Hydrosilylation to Construct Tetrahydroquinoxalines. Green Chem. 2018, 20, 403-411.
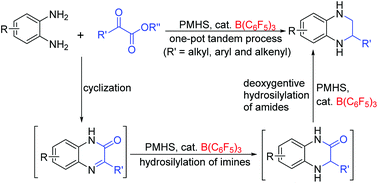
196. R. Begum, Z. H. Farooqi, et al. Applications of UV/Vis Spectroscopy in Characterization and Catalytic Activity of Noble Metal Nanoparticles Fabricated in Responsive Polymer Microgels: A Review. Crit. Rev. Anal. Chem. 2018, 48, 503-516.
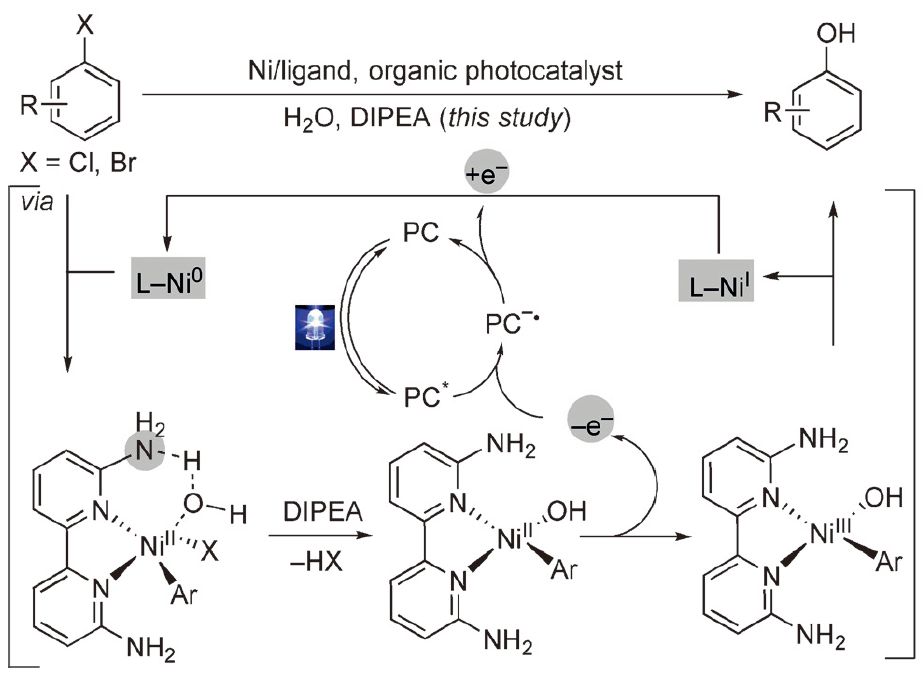
195. L. Yang, Z. Huang, et al. Synthesis of Phenols: Organophotoredox/Nickel Dual Catalytic Hydroxylation of Aryl Halides with Water, Angew. Chem. Int. Ed. 2018, 57, 1968-1972.
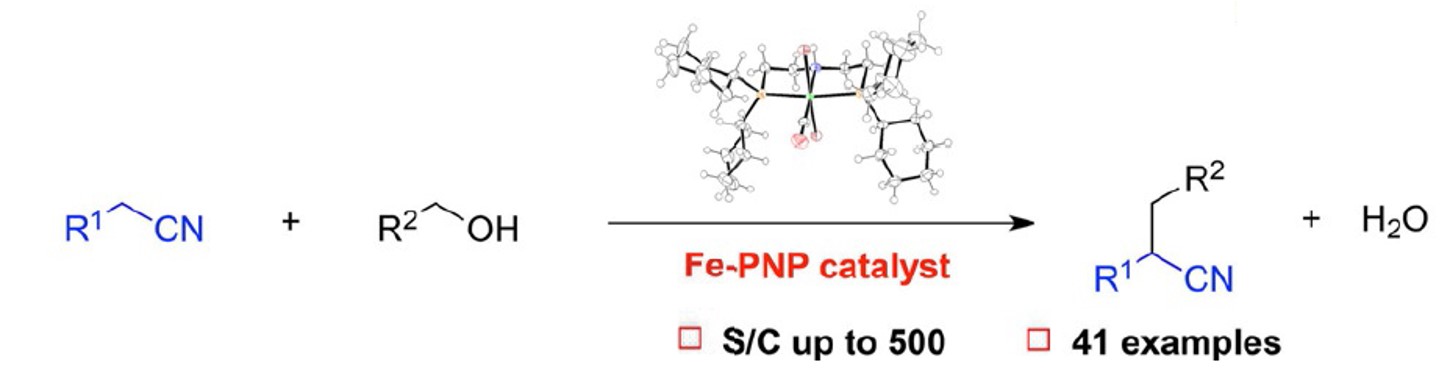
194. W. Ma, S. Cui, et al. Iron-Catalyzed Alkylation of Nitriles with Alcohols, Chem. Eur. J. 2018, 24, 13118-13123.
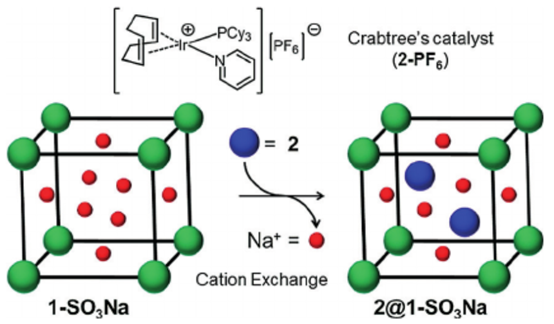
193. A. Grigoropoulos, A. I. McKay, et al. Encapsulation of Crabtree's Catalyst in Sulfonated MIL-101(Cr):Enhancement of Stability and Selectivity between Competing Reaction Pathways by the MOF Chemical Microenvironment, Angew. Chem. Int. Ed. 2018, 57, 4532-4537.
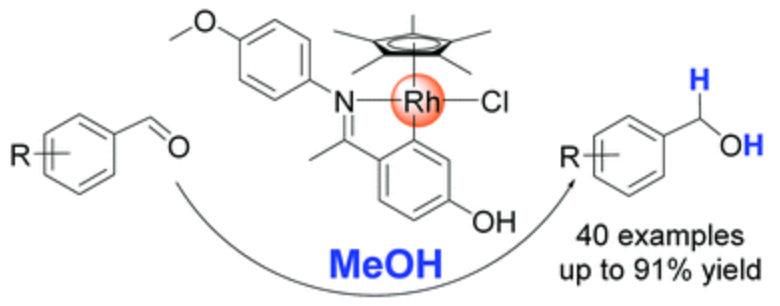
192. A. H. Aboo, E. L. Bennett, et al. Methanol as Hydrogen Source: Transfer Hydrogenation of Aromatic Aldehydes with a Rhodacycle, Chem. Commun. 2018, 54, 11805-11808.
191. G. Zhou, W. Chen, J. Xiao, N,O- vs N,C-Chelation in Half-Sandwich Iridium Complexes: A Dramatic Effect on Enantioselectivity in Asymmetric Transfer Hydrogenation of Ketones, ACS Catal. 2018, 8, 8020-8026.
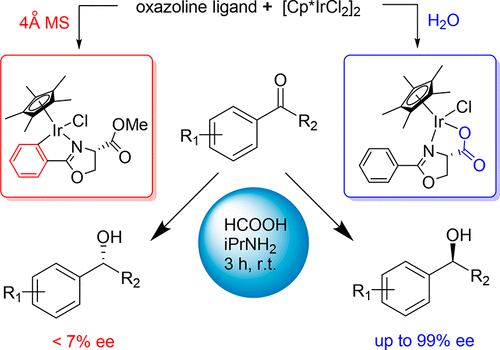
190. J. Chen, M. Zhu,et al. Chemoselective Dehydrogenative Esterification of Aldehydes and Alcohols with a Dimeric Rhodium(II) Catalyst, Chem. Sci. 2017, 8, 6692.
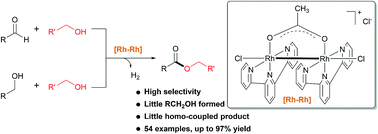
189. X. Jiang, W. Tang, et al. Divergent Dehydrogenative Coupling of Indolines with Alcohols, ACS Catal. 2017, 7, 1831-1835.
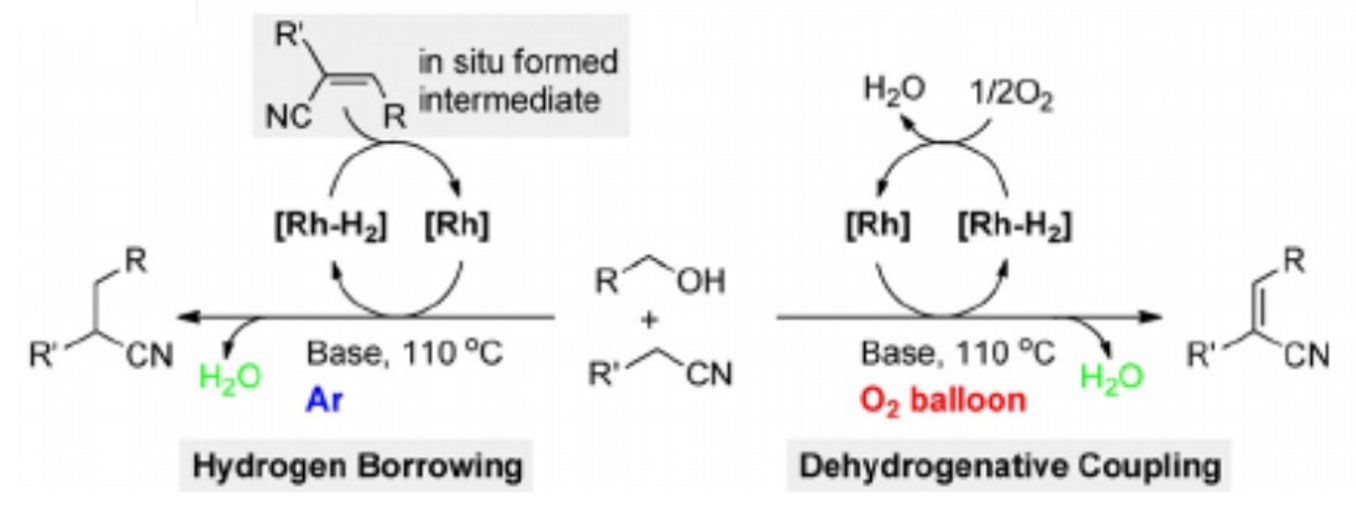
188. J. Li, Y. Liu, et al. Atmosphere-Controlled Chemoselectivity: Rhodium-Catalyzed Alkylation and Olefination of Alkylnitriles with Alcohols, Chem. Eur. J. 2017, 23, 14445-14449.
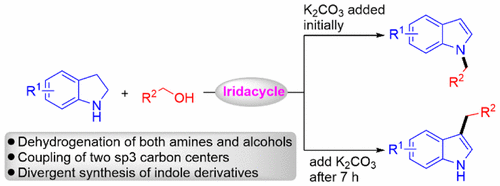
187. H. Liu, J. Chen, et al. Immobilization of Cyclometalated Iridium Complex onto Multiwalled Carbon Nanotubes for Dehydrogenation of Indolines in Aqueous Solution, Ind. Eng. Chem. Res. 2017, 11413–11421.
186. Y. Liu, C. Wang, et al. Reactions Catalysed by a Binuclear Copper Complex: Aerobic Cross Dehydrogenative Coupling of N-Aryl Tetrahydroisoquinolines, Chem. Eur. J. 2017, 23, 3051-3061.
185. Y. Liu, C. Wang, et al. Reactions Catalysed by a Binuclear Copper Complex: Relay Aerobic Oxidation of N-Aryl Tetrahydroisoquinolines to Dihydroisoquinolones with a Vitamin B1 Analogue, Chem. Eur. J. 2017, 23, 3062-3066.
184. Y. Liu, D. Xue, et al. Reactions catalyzed by a Binuclear Copper Complex: Selective Oxidation of Alkenes to Carbonyls with O2, Catal. Sci. Technol. 2017, 7, 5510-5514.
183. C. Wang, J. Xiao, Iridacycles for Hydrogenation and Dehydrogenation Reactions, Chem. Commun. 2017, 53, 3399-3411.
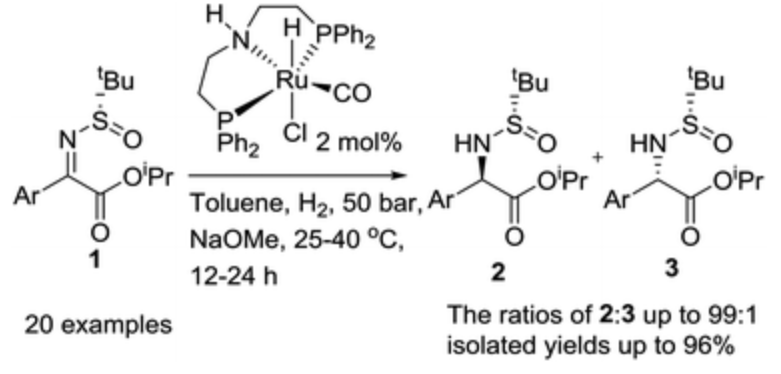
182. Q. Wei, F. Zhang, et al. Ru-Catalyzed Highly Diastereoselective Hydrogenation of N-tert-Butylsulfinyl Ketimines for the Synthesis of Aryl Glycine Derivatives, Org. Biomol. Chem. 2017, 15, 5468-5471.
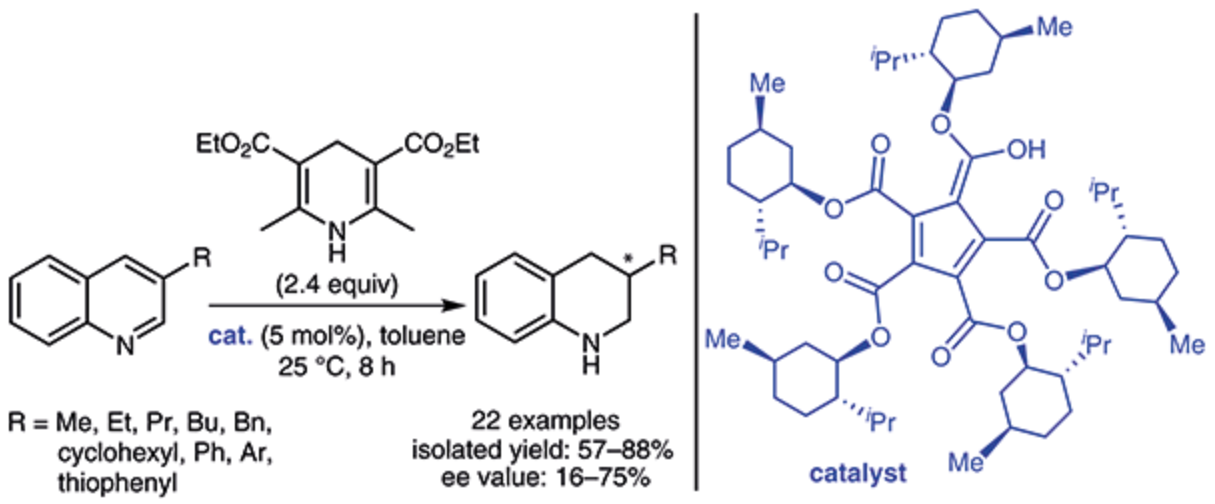
181. X. Zhao, J. Xiao, W. Tang, Enantioselective Reduction of 3-Substituted Quinolines with a Cyclopentadiene-Based Chiral Bronsted Acid. Synthesis 2017, 49, 3157-3164.
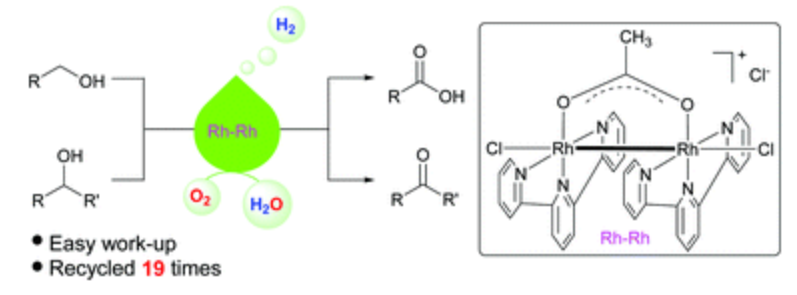
180. X. Wang, C. Wang, et al. Acceptorless dehydrogenation and aerobic oxidation of alcohols with a reusable binuclear rhodium(II) catalyst in water. Green Chem. 2016, 18, 4605-4610.
179. W. Zhou, T. Taboonpong, et al. A Convenient Procedure for the Oxidative Dehydrogenation of N-Heterocycles Catalyzed by FeCl2/DMSO. Synlett 2016, 27, 1806-1809.

178. X. Wu, C. Wang, J. Xiao, Transfer Hydrogenation in Water. Chem. Rec. 2016, 16, 2768-2782.
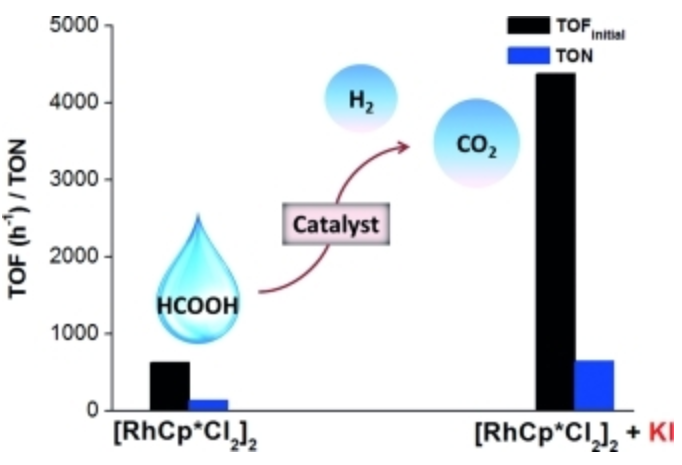
177. Z. Wang, S. Liu, et al. Iodide-Promoted Dehydrogenation of Formic Acid on a Rhodium Complex. Eur. J. Inorg. Chem. 2016, 490-496.
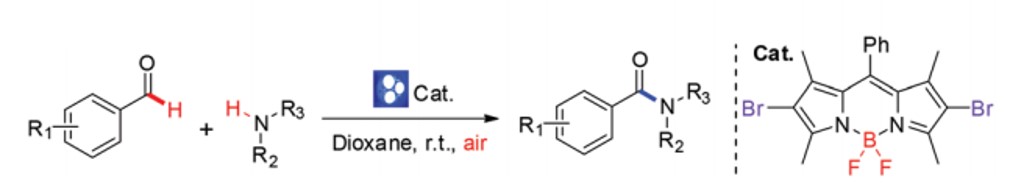
176. X. Wang, S. Yu, et al. BODIPY Catalyzed Amide Synthesis Promoted by BHT and Air under Visible Light. Org. Biomol. Chem. 2016, 14, 7028.
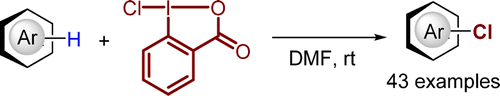
175. M. Wang, Y. Zhang, et al. Story of an Age-Old Reagent: An Electrophilic Chlorination of Arenes and Heterocycles by 1-Chloro-1,2-benziodoxol-3-one. Org. Lett. 2016, 18, 1976−1979.
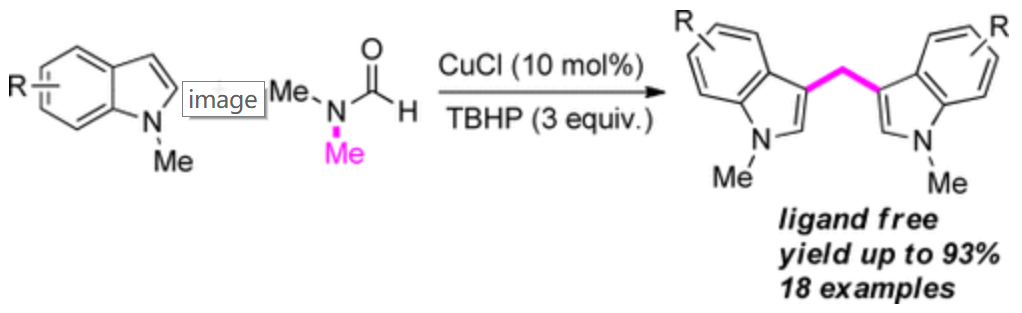
174. F. Pu, Y. Li, et al. Copper-Catalyzed Coupling of Indoles with Dimethylformamide as a Methylenating Reagent. Adv. Synth. Catal. 2016, 358, 539 –542.
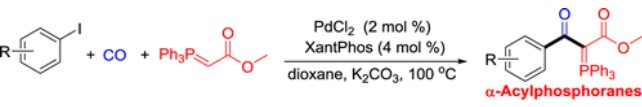
173. X. Guo, W. Ma, et al. Palladium-Catalyzed Ylidyl-Carbonylation of Aryl Halides to Produce α-Acylphosphoranes. Org. Lett. 2016, 18, 4824−4827.
172. S. Gülcemal, D. Gülcemal, et al. Acceptorless Dehydrogenative Oxidation of Secondary Alcohols Catalysed by Cp*IrIII–NHC Complexes. Chem. Eur. J. 2016, 22, 10513 –10522.
171. A. Grigoropoulos, G. F. S. Whitehead, et al. Encapsulation of an organometallic cationic catalyst by direct exchange into an anionic MOF. Chem. Sci. 2016, 7, 2037-2050.
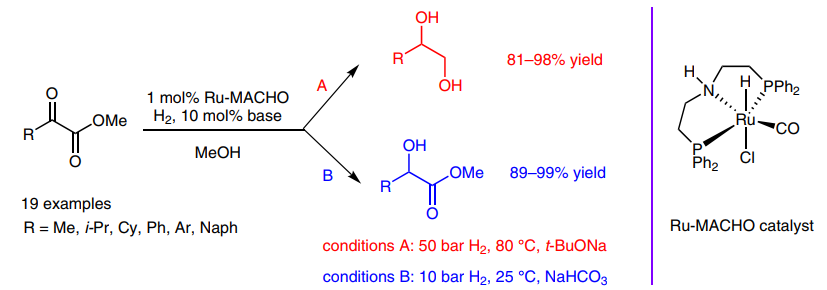
170. S. Gao, W. Tang, et al. Ru-MACHO-Catalyzed Highly Chemoselective Hydrogenation of a-Keto Esters to 1,2-Diols or α-Hydroxy Esters. Synlett 2016, 27, 1748-1752.
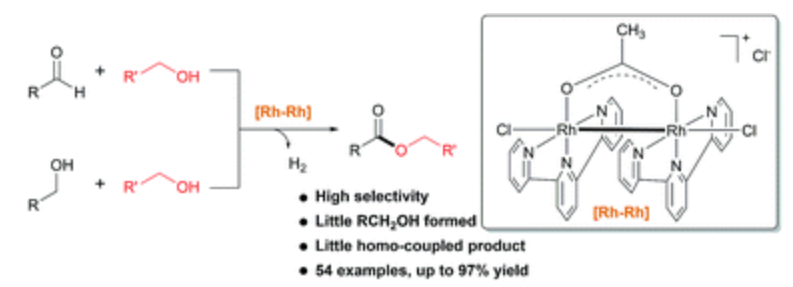
169. J. Chen, M. Zhu, et al. Chemoselective Dehydrogenative Esterification of Aldehydes and Alcohols with a Dimeric Rhodium(II) Catalyst. Chem. Sci. 2016, 7, 4428-4434.
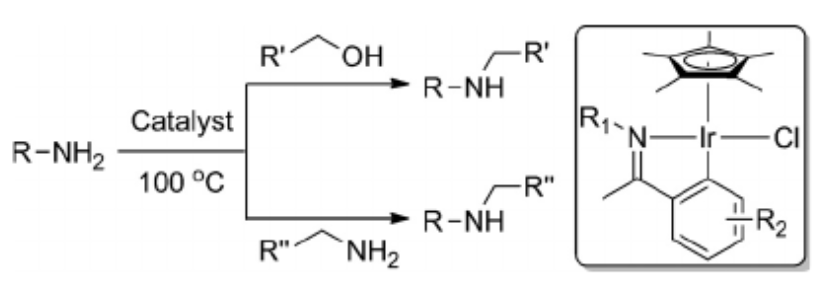
168.Q. Zhou, C. Wang, et al. Alkylation of Amines with Alcohols and Amines by a Single Catalyst under Mild Conditions. Chem. Eur. J. 2015, 21, 9656 –9661.
167. M. Zhu, C. Wang, et al. Transition-Metal-Free Synthesis of Quinolines from 2-Nitrobenzyl Alcohol in Water. Tetrahedron Lett. 2015, 56, 6758–6761.
166. Y. Wei, X. Wu, et al. Transfer Hydrogenation in Aqueous Media. Catal. Today 2015, 247, 104–116.
165. B. Villa‐Marcos, J. Xiao, Metal and Organo-Catalysed Asymmetric Hydroaminomethylation of Styrenes. Chinese J. Catal. 2015, 36, 106–112.
164. D. Talwar, H. Y. Li, et al. A Simple Iridicycle Catalyst for Efficient Transfer Hydrogenation of N-Heterocycles in Water. Chem. Eur. J. 2015, 21, 5370 –5379.
163. D. Talwar, A. Gonzalez‐de‐Castro, et al. Regioselective Acceptorless Dehydrogenative Coupling of N-Heterocycles toward Functionalized Quinolines, Phenanthrolines, and Indoles. Angew. Chem. 2015, 127, 5312-5316.
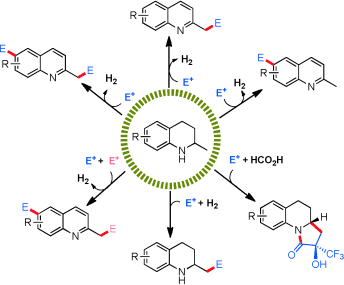
162. W. Ma, D. Xue, et al. Carbonylative Coupling of Allylic Acetates with Aryl Boronic Acids. Chem. Commun. 2015, 51, 8797-8800.
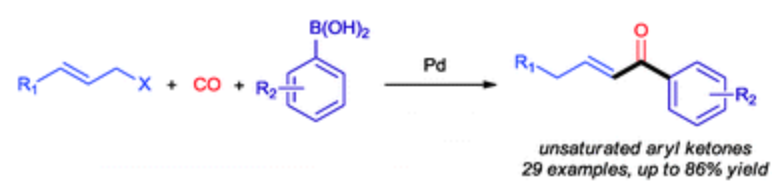
161. D. Gülcemal, S. Gülcemal, et al. A New Phenoxide Chelated IrIII N-Heterocyclic Carbene Complex and Its Application in Reductive Amination Reactions. Organometallics 2015, 34, 4394–4400.
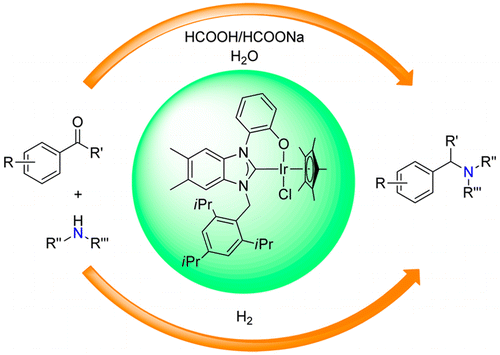
160. A. Gonzalez-de-Castro, J. Xiao, Green and Efficient: Iron-Catalyzed Selective Oxidation of Olefins to Carbonyls with O2. J. Am. Chem. Soc. 2015, 137, 8206–8218.
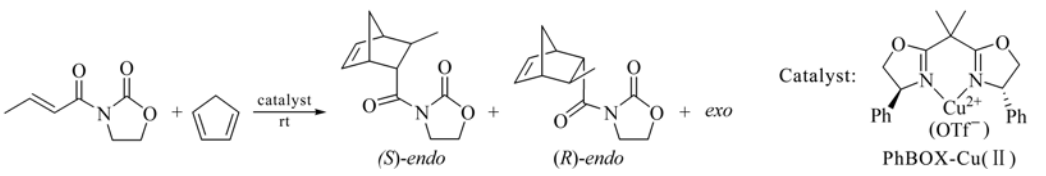
159. C. Zhao, D. Xue, et al. Methanol-Promoted Borylation of Arylamines: a Simple and Green Synthetic Method to Arylboronic Acids and Arylboronates. Synlett 2014, 25, 1577-1584.
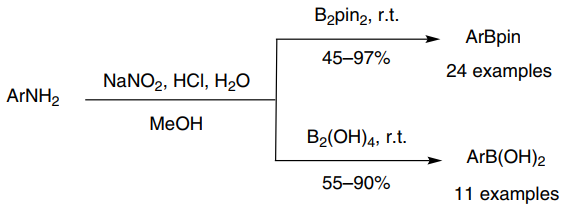
158. D. Xue, Z.-H. Jia, et al. Direct Arylation of N-Heteroarenes with Aryldiazonium Salts by Photoredox Catalysis in Water. Chem. Eur. J. 2014, 20, 2960-2965.
157. B. Wu, C. Wang, J. Xiao, Rapid Synthesis of Water-Soluble Carbon Nanotubes-Supported PtRu Nanoparticles for Methanol Electrooxidation. Diam. Relat. Mater. 2014, 46, 1–7.
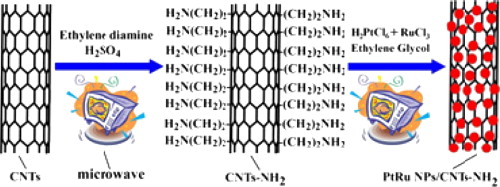
156. B. Wu, C. Wang, et al. High Aqueous Solubility of Carboxylated-Carbon Nanotubes as Support for PtRu Nanoparticles: Enhanced Dispersion and Electrocatalytic Performance. Int. J. Hydrog. Energy. 2014, 39, 7318-7325.
155. Y. Wei, J. Wu, et al. Highly Efficient Rhodium-Catalyzed Transfer Hydrogenation of Nitroarenes into Amines and Formanilides. Synlett 2014, 25, 1295-1298.
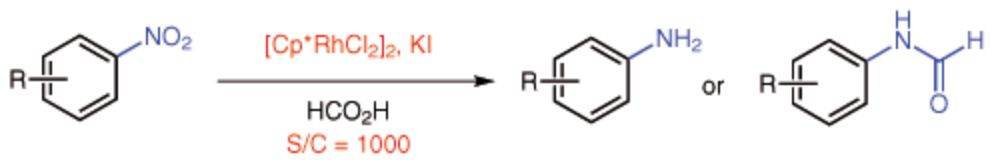
154. Y. Wei, C. Wang, et al. Catalyst-Free Transformation of Levulinic Acid into Pyrrolidinones with Formic Acid. Green Chem. 2014, 16, 1093-1096.
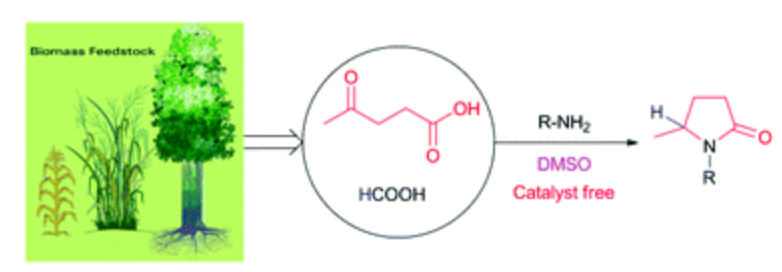
153. J.-D. Wang,Y.-X. Liu, et al. Amination of Benzoxazoles by Visible-Light Photoredox Catalysis. Synlett 2014, 25, 2013-2018.

152. C. Wang, J. Xiao, Asymmetric Reductive Amination. Top. Curr. Chem. 2014, 343, 261-282.
151. W. Tang, J. Xiao, Asymmetric Hydrogenation of Imines via Metal-Organo Cooperative Catalysis. Synthesis 2014, 46, 1297-1302.
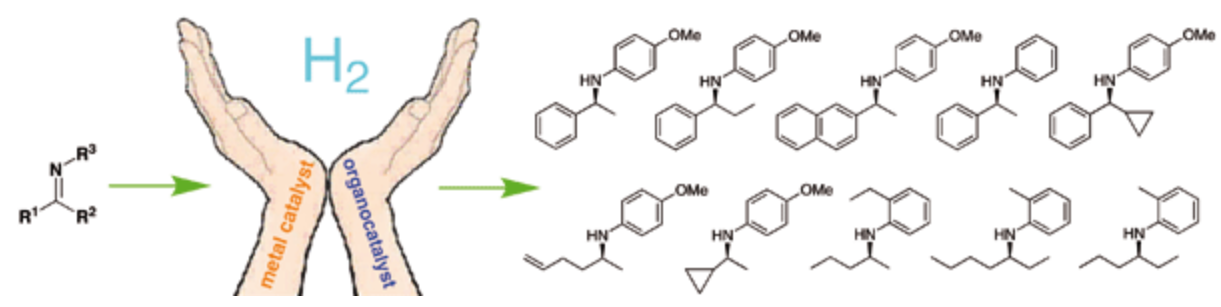
150. W. Tang, C. Lau, et al. Cyclometalated Iridium Complexes as Highly Active Catalysts for the Hydrogenation of Imines. Synlett 2014, 25, 81-84.
149. D. Talwar, N. P. Salguero, et al. Primary Amines by Transfer Hydrogenation Reductive Amination of Ketones by Using Cyclometalated IrIII Catalysts. Chem. Eur. J. 2014, 20, 245-252.
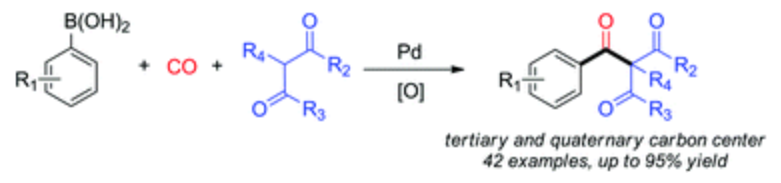
148. W. Lu, Y. Li, et al. Pd-Catalyzed Carbonylation for the Construction of Tertiary and Quaternary Carbon Centers with Sp3 Carbon Partners. Org. Biomol. Chem. 2014, 12, 5243-5249.
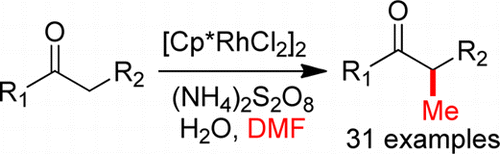
147. Y. Li, D. Xue, et al. DMF as Carbon Source: Rh-Catalyzed α-Methylation of Ketones. Org. Lett. 2014, 16, 66–69.
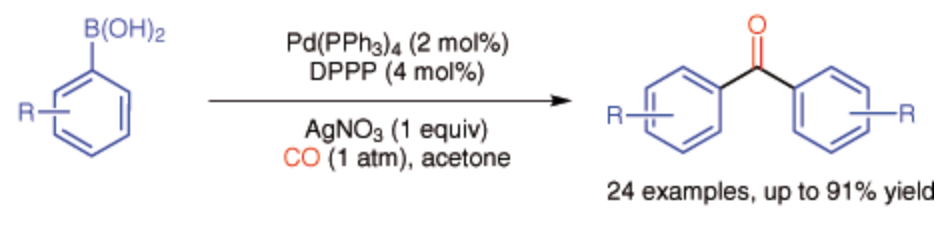
146. Y. Li, W. Lu, et al. Palladium-Catalyzed Oxidative Carbonylation for the Synthesis of Symmetrical Diaryl Ketones at Atmospheric CO Pressure. Synlett 2014, 25, 1097-1100.
145. X. Jiang, C. Wang, et al. A General Method for N-Methylation of Amines and Nitro Compounds with Dimethylsulfoxide. Chem. Eur. J. 2014, 20, 58 – 63.
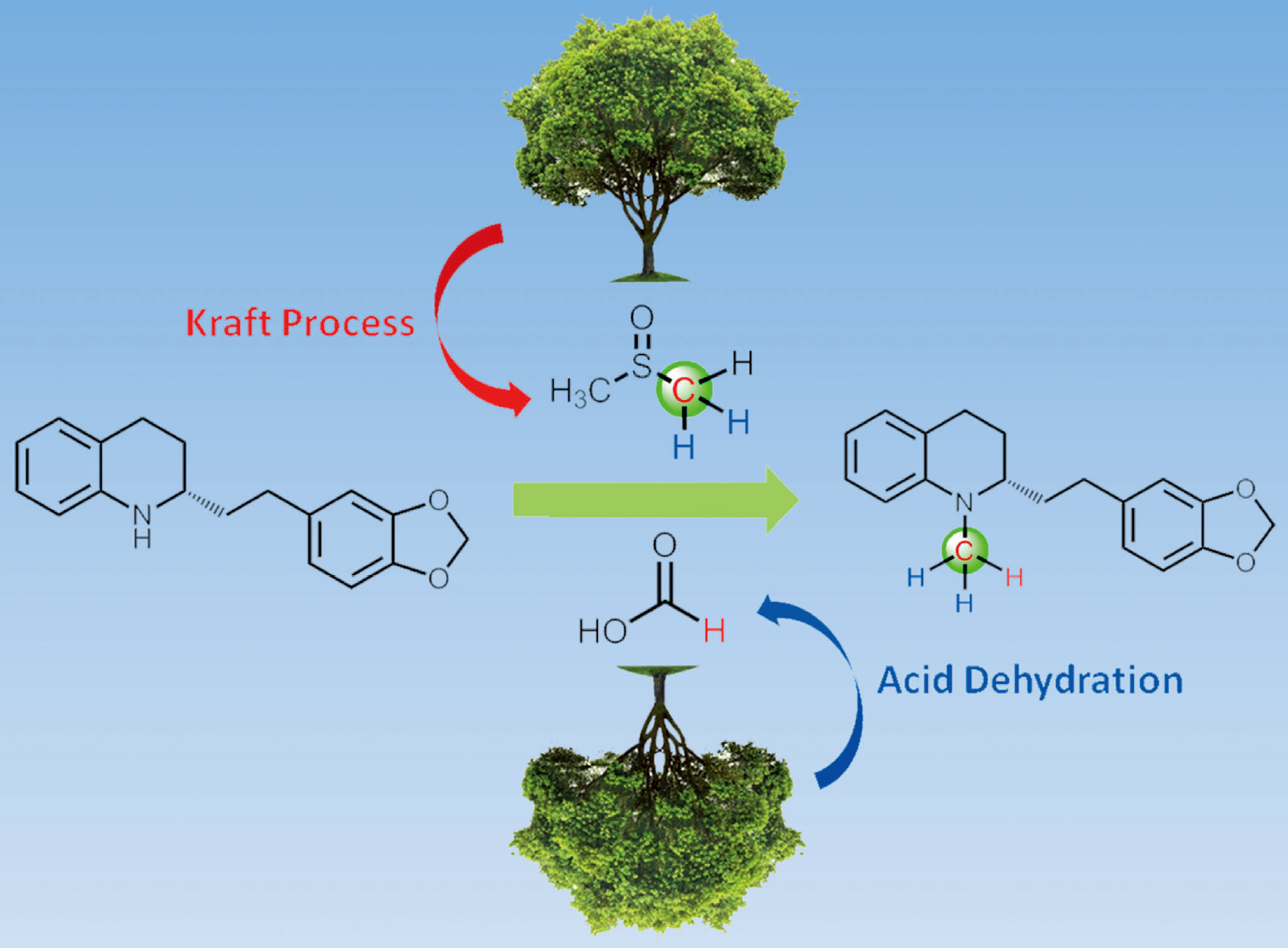
144. A. Gonzalez-de-Castro, C. M. Robertson, J. Xiao, Dehydrogenative α-Oxygenation of Ethers with an Iron Catalyst. J. Am. Chem. Soc. 2014, 136, 8350–8360.
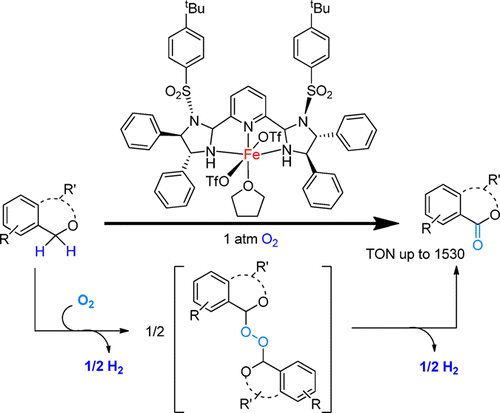
143. D. Talwar, N. P. Salguero, et al. Primary Amines by Transfer Hydrogenative Reductive Amination of Ketones by Using Cyclometalated IrIII Catalysts. Chem. Eur. J. 2014, 20, 245-252.
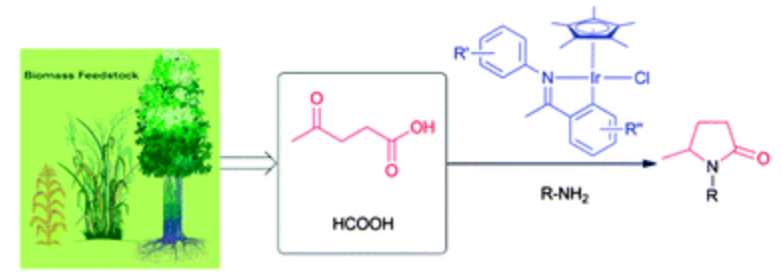
142.Y. Wei, C. Wang, et al. Highly Efficient Transformation of Levulinic Acid into Pyrrolidinones by Iridium Catalysed Transfer Hydrogenation. Chem. Commun. 2013, 49, 5408-5410.
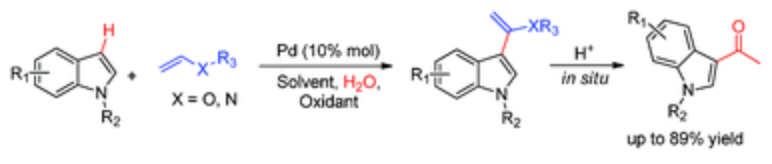
141. Y. Li, D. Xue, et al. 3-Acylindoles via Palladium-Catalysed Regioselective Arylation of Electron-Rich Olefins with Indoles. RSC Adv. 2013, 3, 11463-11466.
140. J. Wu, J. H. Barnard, et al. Robust Cyclometallated Ir(III) Catalysts for the Homogeneous Hydrogenation of N-Heterocycles under Mild Conditions. Chem. Commun. 2013, 49, 7052-7054.
139. L. Zhang, C. Dong, et al. Palladium-Catalysed Regioselective and Stereoselective Oxidative Heck Arylation of Allylamines with Arylboronic Acids. Adv. Syn. Catal. 2013, 355, 1570 – 1578.
138. S.-Z. Nie, X. Sun, et al. Unprecedented Construction of C=C Double Bonds via Ir-Catalysed Dehydrogenative and Dehydrative Cross-Couplings. Org. Lett. 2013, 15, 2394–2397.
137. W. Tang, S. Johnston, et al. Cooperative Catalysis: Combining an Achiral Metal Catalyst with a Chiral Bronsted Acid Enables Highly Selective Hydrogenation of Imines. Chem. Eur. J. 2013, 19, 14187 – 14193.
136. J. Wu, D. Talwar, et al. Acceptorless Dehydrogenation of Nitrogen Heterocycles with a Versatile Iridium Catalyst. Angew. Chem. Int. Ed. 2013, 52, 6983 –6987.
135. Q. Lei, Y. Wei, et al. Fast Reductive Amination by Transfer Hydrogenation "on Water". Chem. Eur. J. 2013, 19, 4021-4029.
134. Y.-X. Xia, D. Xue, et al. Room-Temperature Arylation of Arenes and Heteroarenes with Diaryliodonium Salts by Photoredox Catalysis. Synlett, 2013, 24, 507-513.
133. Y. Wei, D. Xue, et al. Cyclometalated Iridium Complexes for Transfer Hydrogenation of Carbonyl Groups in Water. Green Chem. 2013,15, 629-634.
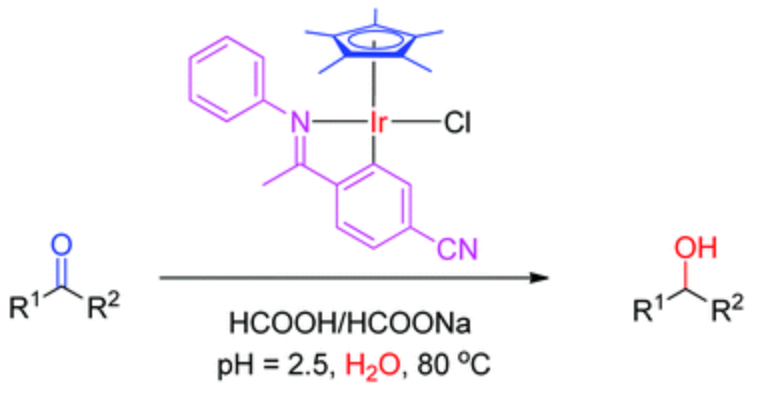
132. C. Wang, H. Y. T. Chen, et al. Synthesis and X-ray Structures of Cyclometalated Iridium Complexes Including the Hydrides. Dalton Trans. 2013, 42, 935-940.
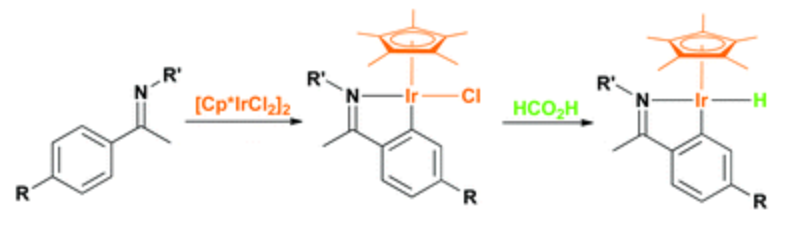
131. W. Tang, S. Johnston, et al. Cooperative Catalysis through Noncovalent Interactions. Angew. Chem. Int. Ed. 2013, 52, 1668 –1672.
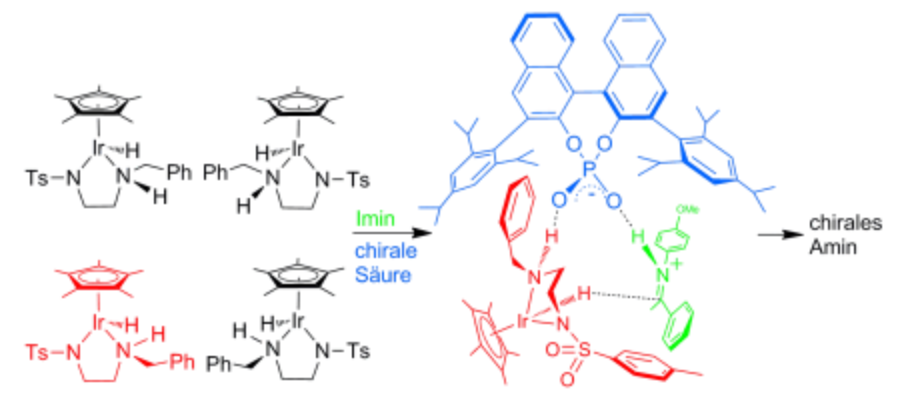
130. J. Wu, W. Tang, et al. Efficient and Chemoselective Reduction of Pyridines to Tetrahydropyridines and Piperidines via Rhodium‐Catalyzed Transfer Hydrogenation. Adv. Synth. Catal. 2013, 355, 35–40.
129.J. H. Barnard, C. Wang, et al. Long-Range Metal–Ligand Bifunctional Catalysis: Cyclometallated Iridium Catalysts for the Mild and Rapid Dehydrogenation of Formic Acid. Chem. Sci. 2013, 4, 1234–1244.
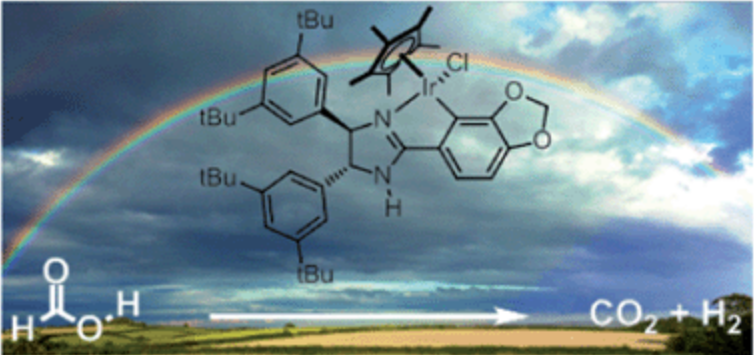
128.J. Wu, C. Wang, et al. The Remarkable Effect of a Simple Ion: Iodide‐Promoted Transfer Hydrogenation of Heteroaromatics. Chem. Eur. J. 2012, 18, 9525-9529.
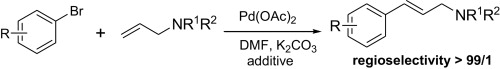
127.Z. Jiang, L. Zhang, et al. Palladium-Catalyzed Highly Regioselective and Stereoselective Arylation of Electron-Rich Allylamines with Aryl Bromides. Tetrahedron. 2012, 68, 4919-4926.
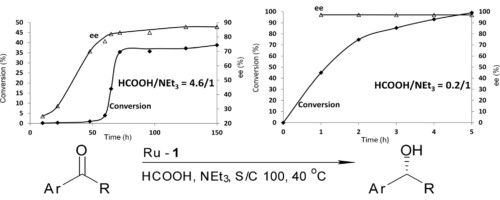
126.N. Martins, N. Mateus, et al. Another Side of the Oxazaphospholidine Oxide Chiral Ortho-Directing Group. Org. Biomol. Chem. 2012, 10, 4036–4042.
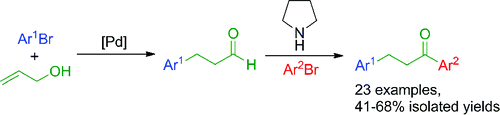
125.P. Colbon, J. H. Barnard, et al. Feeding the Heck Reaction with Alcohol: One-Pot Synthesis of Stilbenes from Aryl Alcohols and Bromides. Adv. Synth. Catal. 2012, 354, 1395-1400.
124.X. Zhou, X. Wu, et al. Varying the Ratio of Formic Acid to Triethylamine Impacts on Asymmetric Transfer Hydrogenation of Ketones. J. Mol. Catal. A-Chem. 2012, 357, 133–140.
123. P. Colbon, J. Ruan, et al. Double Arylation of Allyl Alcohol via a One-Pot Heck Arylation–Isomerization–Acylation Cascade. Org. Lett. 2011, 13, 5456–5459.
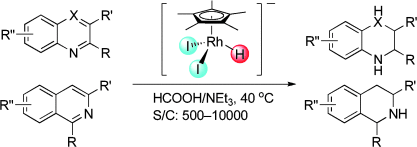
122. J. Tan, W. tang, et al. pH-Regulated Transfer Hydrogenation of Quinoxalines with a Cp*Ir–Diamine Catalyst in Aqueous Media. Tetrahedron. 2011, 67, 6206-6213.
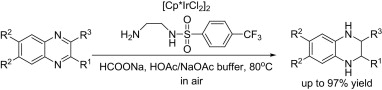
121. C. Wang, B. Villa-Marcosa, J. Xiao, Hydrogenation of Imino Bonds with Half-Sandwich Metal Catalysts. Chem. Commun. 2011, 47, 9773–9785.
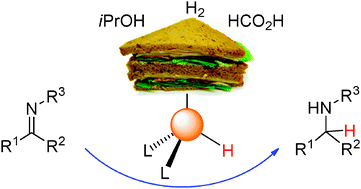
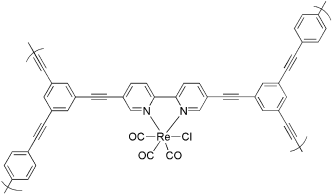
120. J.-X. Jiang, C. Wang, et al. Metal-Organic Conjugated Microporous Polymers. Angew. Chem. 2011, 123, 1104-1107.
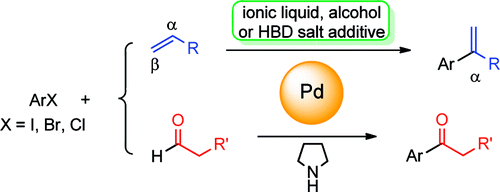
119.J. Ruan, J. Xiao, From α-Arylation of Olefins to Acylation with Aldehydes: A Journey in Regiocontrol of the Heck Reaction. Acc. Chem. Res. 2011, 44, 614–626.
118. J. Ruan, J. A. Iggo, J. Xiao, Direct Synthesis of 1-Indanones via Pd-Catalyzed Olefination and Ethylene Glycol-Promoted Aldol-Type Annulation Cascade. Org. Lett. 2011, 13, 268–271.
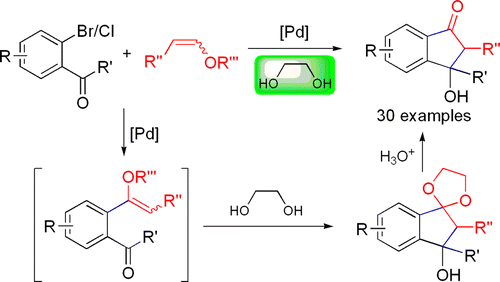
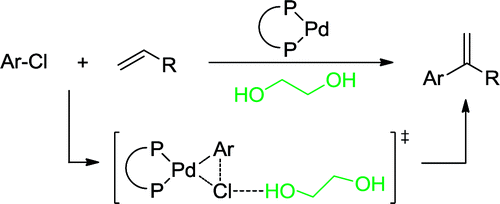
117. J. Ruan, J. A. Iggo, et al. Hydrogen-Bonding-Promoted Oxidative Addition and Regioselective Arylation of Olefins with Aryl Chlorides. J. Am. Chem. Soc. 2010, 132, 16689–16699.
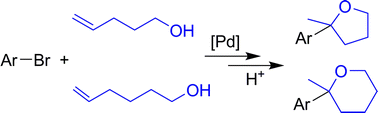
116.M. McConville, J. Ruan, et al. Synthesis of Oxygen Heterocycles by Regioselective Heck Reaction. Org. Biomol. Chem. 2010, 8, 5614–5619.
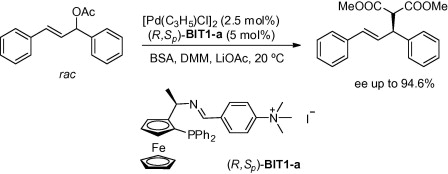
115. H. Yuan, Z. Zhou, et al. Preparation of Quarternary Ammonium Salt-Tagged Ferrocenylphosphine-Imine Ligands and Their Application to Palladium-Catalyzed Asymmetric Allylic Substitution. Tetrahedron Asymmetry. 2010, 21, 1874–1884.
114. C. Wang, A. Pettman, et al. A Versatile Catalyst for Reductive Amination by Transfer Hydrogenation. Angew. Chem. Int. Ed. 2010, 49, 7548 –7552.
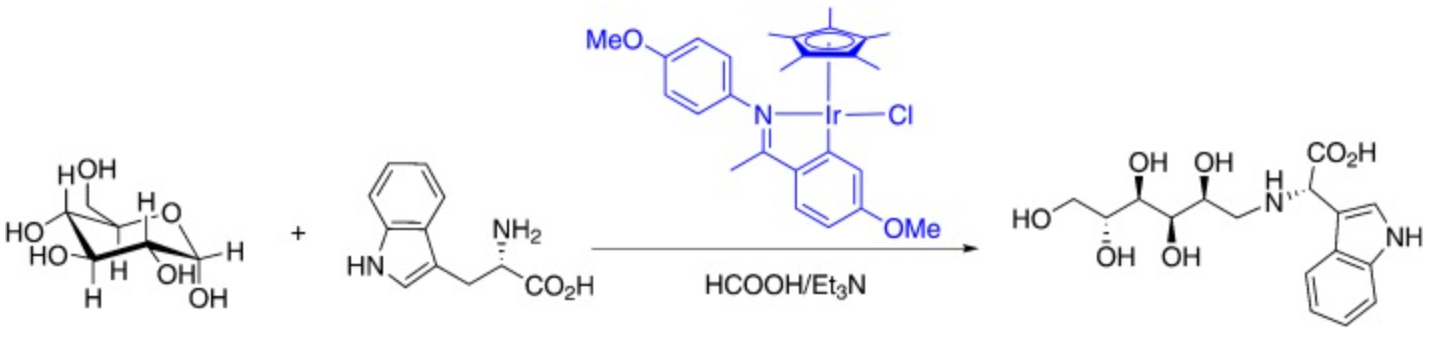
113. P. Colbon, J. Ruan, et al. Direct Acylation of Aryl Chlorides with Aldehydes by Palladium−Pyrrolidine Co-catalysis. Org. Lett. 2010, 12, 3670–3673.
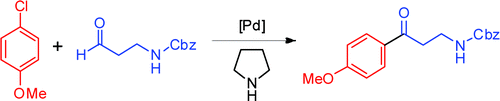
112. M. Liu, Z. Hyder, et al. Efficient Synthesis of Alkyl Aryl Ketones & Ketals via Palladium-Catalyzed Regioselective Arylation of Vinyl Ethers. Org. Biomol. Chem. 2010, 8, 2012-2015.
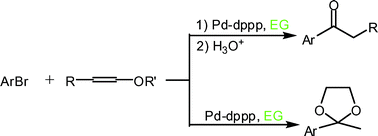
111. X. Wang, C. Wang, J. Xiao, Asymmetric Transfer Hydrogenation in Water with Platinum Group Metal Catalysts. Platinum Metals Rev. 2010, 54, 3-19.
110. B. Villa-Marcos, C. Li, et al. Bifunctional Catalysis: Direct Reductive Amination of Aliphatic Ketones with an Iridium-Phosphate Catalyst. Molecules. 2010, 15, 2453-2472.
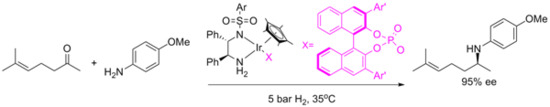
109. M. McConvillea, J. Blacker, J. Xiao, Heck Reaction in Diols and Cascade Formation of Cyclic Ketals. Synthesis 2010, 2, 349-360.
108. C. Wang, C. Li, et al. pH‐Regulated Asymmetric Transfer Hydrogenation of Quinolines in Water. Angew. Chem. Int. Ed. 2009, 48, 6524-6528.
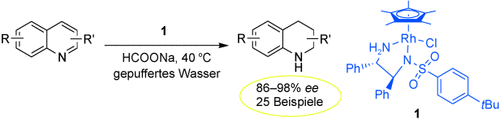
107. J. Ruan, L. Shearer, et al. [2.2]Paracyclophane-Based Monophosphine Ligand for Palladium-Catalyzed Cross-Coupling Reactions of Aryl Chlorides. Org. Biomol. Chem. 2009,7, 3236-3242.
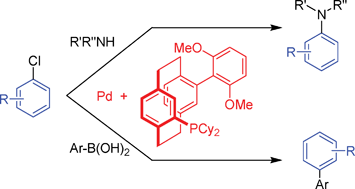
106. C. Li, B. Villa-Marcos, J. Xiao, Metal−Brønsted Acid Cooperative Catalysis for Asymmetric Reductive Amination. J. Am. Chem. Soc. 2009, 131, 6967–6969.
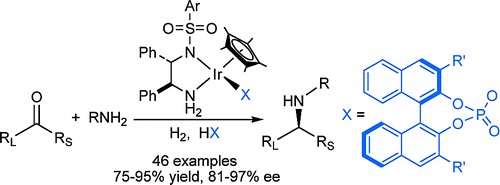
105. O. Saidi, S. Liu, J. Xiao, Effects of Ligands on the Rhodium-Catalyzed Hydroformylation of Acrylate. J. Mol. Catal. Chem. 2009, 305, 130–134.
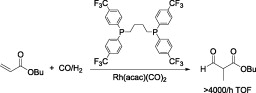
104. S. Liu, O. Saidi, J. Xiao, Electron-Deficient Phosphines Accelerate the Heck Reaction of Electronrich Olefins in Ionic Liquid. Lett. Org. Chem. 2009, 6, 60-64.
103. M. McConville, O. Saidi, et al. Regioselective Heck Vinylation of Electron-Rich Olefins with Vinyl Halides: Is the Neutral Pathway in Operation? J. Org. Chem. 2009, 74, 2692–2698.
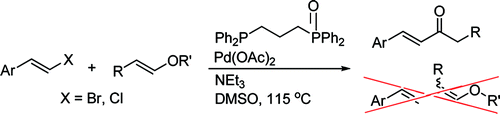
102. D. Vinci, N. Martins, et al. Ferrocenyl Phosphine–Oxazaphospholidine Oxide Ligands for the Suzuki–Miyaura Coupling of Hindered Aryl Bromides and Chlorides. Can. J. Chem. 2009, 87, 171-175.
101. C. Li, C. Wang, et al. Chiral Counteranion-Aided Asymmetric Hydrogenation of Acyclic Imines. J. Am. Chem. Soc. 2008, 130, 14450–14451.
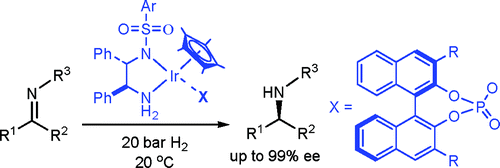
100. C. Li, J. Xiao, Asymmetric Hydrogenation of Cyclic Imines with an Ionic Cp*Rh(III) Catalyst. J. Am. Chem. Soc. 2008, 130, 13208–13209.
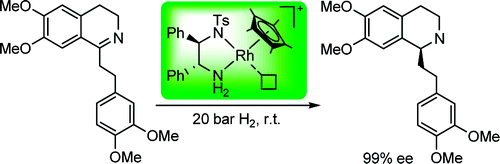
99. C. Wang, X. Wu, J. Xiao, Broader, Greener, and More Efficient: Recent Advances in Asymmetric Transfer Hydrogenation. Chem. Asian J. 2008, 3, 1750 – 1770.
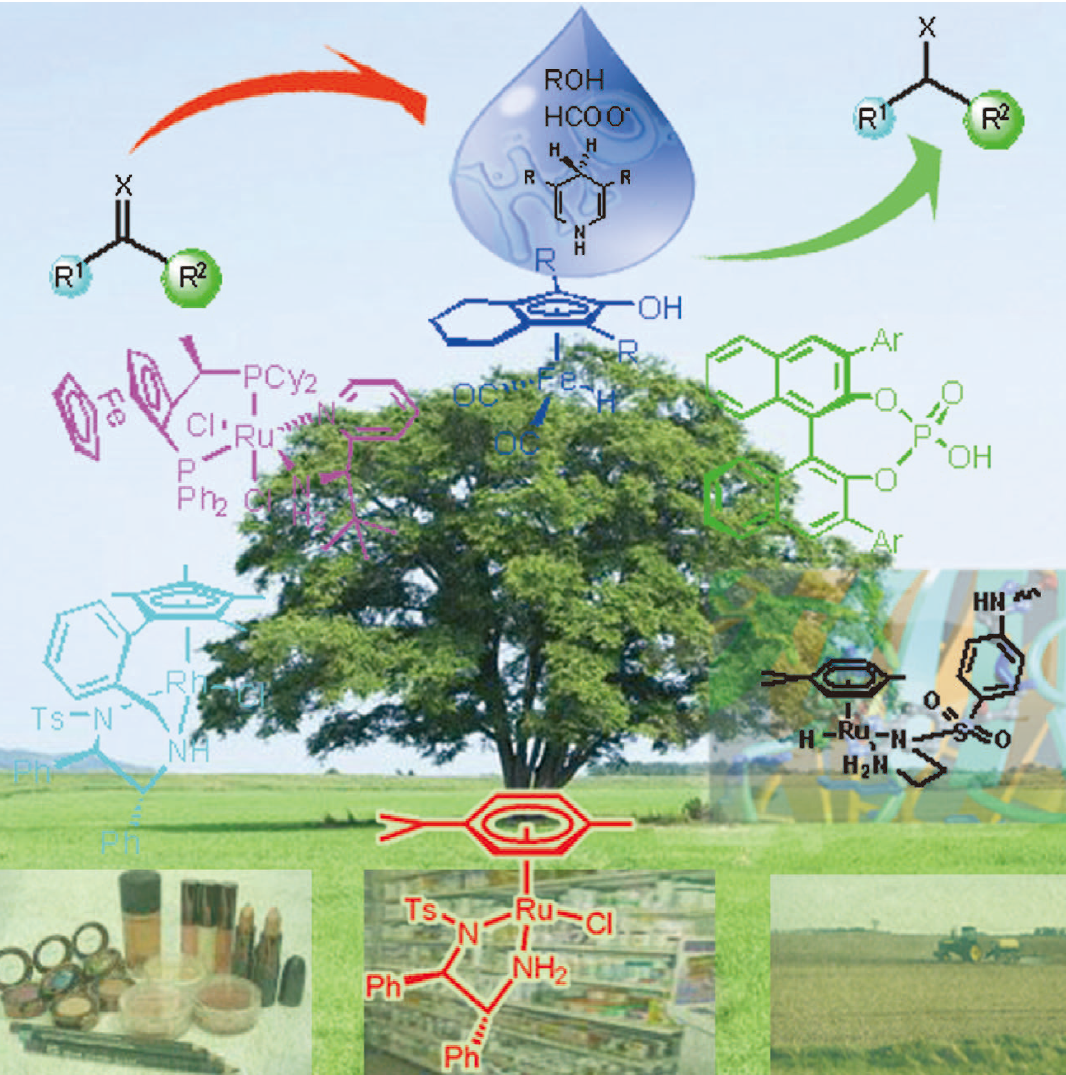
98. D. Xu, Z. Liu, et al. Palladium-Catalyzed Regiocontrolled Internal Heteroarylation of Electron-Rich Olefins with Heteroaryl Halides. Tetrahedron Lett. 2008, 49, 6104–6107.
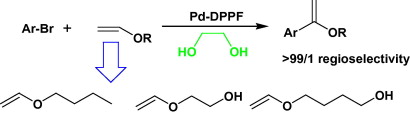
97. J. Ruan, O. Saidi, et al. Direct Acylation of Aryl Bromides with Aldehydes by Palladium Catalysis. J. Am. Chem. Soc. 2008, 130, 10510-10511.
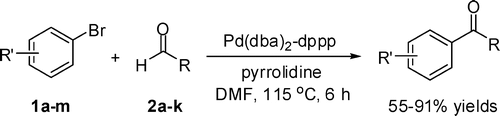
96. X. Wu, J. Liu, et al. A Multilateral Mechanistic Study into Asymmetric Transfer Hydrogenation in Water. Chem. Eur. J. 2008, 14, 7699– 7715.
95. X. Wu, J. Mo, et al. Green Chemistry: C–C Coupling and Asymmetric Reduction by Innovative Catalysis. Prog. Nat. Sci. 2008, 18, 639–652.
94. O. Saidi, J. Ruan, et al. Highly Regioselective Hydroformylation of Enamides with Phosphite Ligands. Tetrahedron Lett. 2008, 49, 3516-3519.
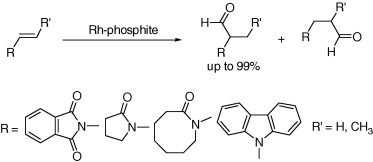
93. Z. Liu, D. Xu, et al. A General Method for Regioselective Heck Arylation of Electron-Rich N-Acyl-N-Vinylamine with Aryl Halides. Tetrahedron Lett. 2008, 49, 2756-2760.
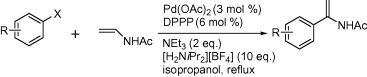
92. Z. Hyder, J. Ruan, J. Xiao, Hydrogen‐Bond‐Directed Catalysis: Faster, Regioselective and Cleaner Heck Arylation of Electron‐Rich Olefins in Alcohols. Chem. Eur. J. 2008, 14, 5555 – 5566.
91. H. Wang, H. Wang, et al. Electronic and Steric Effects of Bis(oxazolinyl)pyridine Ligands on Asymmetric Diels-Alder Reactions. J. Mol. Catalysis. Chem. 2008, 285, 128-131.
90. J. Liu, X. Wu, et al. Half-Sandwich Iridium Complexes-Synthesis and Applications in Catalysis. Coord. Chem. Rev. 2008, 252, 782-809.
89. J. Ruan, X. Li, et al. Oxygen and Base-Free Oxidative Heck Reactions of Arylboronic Acids with Olefins. J. Am. Chem. Soc. 2008, 130, 2424–2425.
88. X. Wu, X. Li, et al. RhIII‐ and IrIII‐Catalyzed Asymmetric Transfer Hydrogenation of Ketones in Water. Chem. Eur. J. 2008, 14, 2209 – 2222.
87. X. Wu, C. Corcoran, et al. A Versatile Iridium Catalyst for Aldehyde Reduction in Water. ChemSusChem 2008, 1, 71–74.
86.S. Liu, N. Thomson, et al. Ionic Liquids as Solvent for Regioselective Arylation of α-Substituted Allylic Alcohols by Aryl Bromides. J. Mol. Catal. Chem. 2008, 279, 210-217.
85. M. Joshaghani, E. Faramarzi, et al. Highly Efficient Suzuki Coupling Using Moderately Bulky Tolylphosphine Ligands. J. Mol.Catal. Chem. 2007, 273, 310–315.
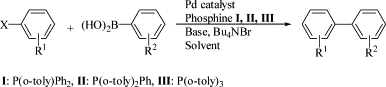
84. L. Zhong, Q. Gao, et al. Direct Catalytic Asymmetric Aldol Reactions on Chiral Catalysts Assembled in the Interface of Emulsion Droplets. J. Catal. 2007, 250, 360-364.
83. L. Zhong, J. Xiao, C. Li, Direct Asymmetric Aldol Reactions on Heterogeneous Bifunctional Catalyst. Chin. J. Catal. 2007, 28, 673–675.
82. S. Liu, J. Xiao, Toward Green Catalytic Synthesis-Transition Metal-Catalyzed Reactions in Non-Conventional Media. J. Mol. Catal. Chem. 2007, 270, 1–43.
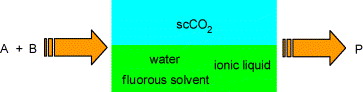
81. X. Wu, J. Xiao, Aqueous-Phase Asymmetric Transfer Hydrogenation of Ketones-A Greener Approach to Chiral Alcohols. Chem. Commun. 2007, 2449-2466.
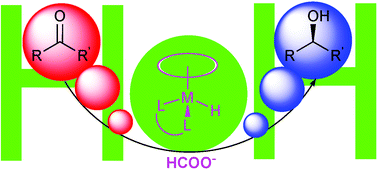
80. M. Joshaghani, M. Daryanavard, et al. A Highly Efficient Catalyst for Suzuki Coupling of Aryl Halides and Bromoary-l Phosphine Oxides. Tetrahedron Lett. 2007, 48, 2025-2027.
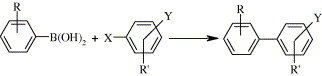
79. J. Mo, J. Ruan, et al. Palladium-Catalyzed Heck Arylation of 5-Hexen-2-one in Ionic Liquid: A Novel Approach to Arylated Gamma, Delta-Unsaturated Ketones. J. Mol. Catal. Chem. 2007, 261, 267-275.
78. W. Hong, L. Jian, et al. Asymmetric Diels-Alder Reactions on Supported Bis(oxazoline) Catalysts. Chin. J. Catal. 2006, 27, 946–948.
77. M. Joshaghani, E. Faramarzi, et al. Efficient Suzuki Cross-Coupling Reactions Using Bulky Phosphines. J. Mol. Catal. Chem. 2006, 259, 35–40.

76. X. Wu, J. Liu, et al. On Water and in Air: Fast and Highly Chemoselective Transfer Hydrogenation of Aldehydes with Iridium Catalysts. Angew. Chem. Int. Ed. 2006, 45, 6718 –6722.
75. D. Vinci, X. Wu, et al. Synthesis of 2-Diphenylphosphinoyl-2-Halo Biphenyls Via Suzuki-Miyaura Coupling as Possible Route to Non-Symmetric Biphenyl Phosphines. Lett. Org. Chem. 2006, 3, 567-570.
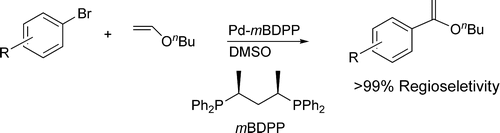
74. S. Liu, N. Berry, et al. Pd−mBDPP-Catalyzed Regioselective Internal Arylation of Electron-Rich Olefins by Aryl Halides. J. Org. Chem. 2006, 71, 7467–7470.
73. Z. Hyder, J. Mo, J. Xiao, Palladium‐Catalysed Direct Regioselective Synthesis of Cyclic Ketals from Electron‐Rich Olefins and Aryl Bromides in Ionic Liquidsarticl. Adv. Synth. Catal. 2006, 348, 1699 – 1704.
72. J. Mo, L. Xu, et al. Regioselective Heck Arylation of Unsaturated Alcohols by Palladium Catalysis in Ionic Liquid. Chem. Commun. 2006, 3591-3593.
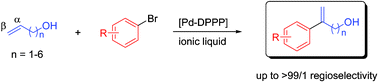
71. J. Mo, J. Xiao, The Heck Reaction of Electron‐Rich Olefins with Regiocontrol by Hydrogen‐Bond Donors. Angew. Chem. 2006, 118, 4258 –4263.
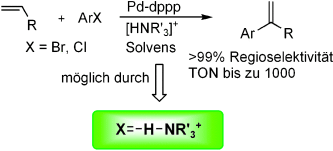
70. X. Li, J. Blacker, et al. An Efficient Ir (III) Catalyst for the Asymmetric Transfer Hydrogenation of Ketones in Neat Water. Synlett 2006, 8, 1155-1160.
69. X. Wu, X. Li, et al. β-Amino Alcohols as Ligands for Asymmetric Transfer Hydrogenation of Ketones in Water. J. Mol. Catal. Chem. 2006, 247, 153–158.
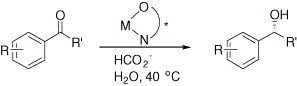
68. H. Wang, X. Liu, et al. Asymmetric Diels–Alder Reactions with Hydrogen Bonding Heterogeneous Catalysts and Mechanistic Studies on the Reversal of Enantioselectivity. Tetrahedron 2006, 62, 1025–1032.
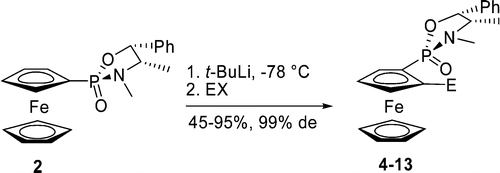
67. D. Vinci, N. Mateus, et al. Oxazaphospholidine-oxide as an Efficient ortho-Directing Group for the Diastereoselective Deprotonation of Ferrocene. Org. Lett. 2006, 8, 215–218.
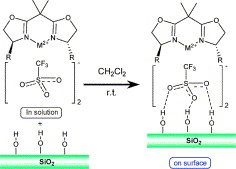
66. J. Mo, S. Liu, J. Xiao, Palladium-Catalyzed Regioselective Heck Arylation of Electron-Rich Olefins in a Molecular Solvent-Ionic Liquid Cocktail. Tetrahedron 2005, 61, 9902–9907.
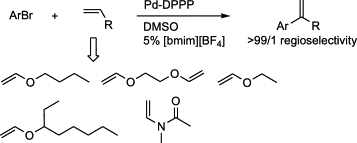
65. X. Wu, D. Vinci, et al. A Remarkably Effective Catalyst for the Asymmetric Transfer Hydrogenation of Aromatic Ketones in Water and Air. Chem. Commun. 2005, 4447-4449.
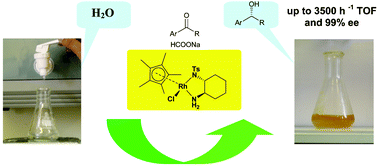
64. W. Pei, J. Mo, J. Xiao, Highly Regioselective Heck Reactions of Heteroaryl Halides with Electron-Rich Olefins in Ionic Liquid. J. Organometal. Chem. 2005, 690, 3546–3551.
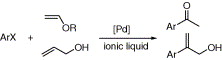
63. H. Zhang, S. Xiang, et al. Heterogeneous Enantioselective Epoxidation Catalyzed by Mn(salen) Complexes Grafted onto Mesoporous Materials by Phenoxy Group. J. Mol. Catal. Chem. 2005, 238, 175–184.
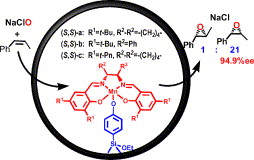
62. X. Wu, X. Li, et al. Insight into and Practical Application of pH‐Controlled Asymmetric Transfer Hydrogenation of Aromatic Ketones in Water. Angew. Chem. 2005, 117, 3473-3477.
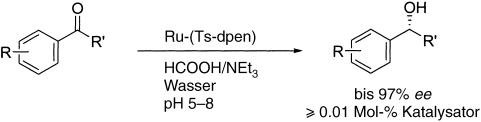
61. J. Mo, L. Xu, J. Xiao, Ionic Liquid-Promoted, Highly Regioselective Heck Arylation of Electron-Rich Olefins by Aryl Halides. J. Am. Chem. Soc. 2005, 127, 751–760.
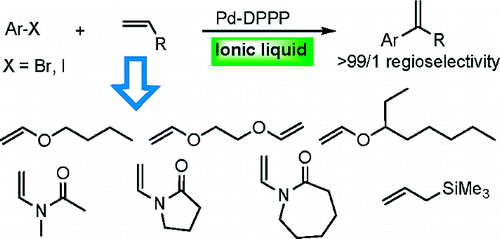
60. R. Stead, J. Xiao, A New Class of Ferrocenyl Phosphines. Lett. Org. Chem. 2004, 1, 148-150.
59. C. Baillie, L. Zhang, J. Xiao, Ferrocenyl Monophosphine Ligands: Synthesis and Applications in the Suzuki-Miyaura Coupling of Aryl Chlorides. J. Org. Chem. 2004, 69, 7779–7782.
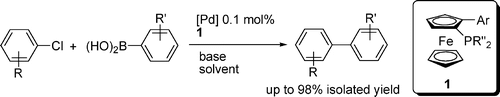
58. X. Li, X. Wu, et al. Asymmetric Transfer Hydrogenation in Water with a Supported Noyori−Ikariya Catalyst. Org. Lett. 2004, 6, 3321–3324.
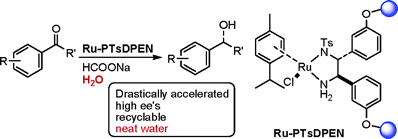
57. C. Baillie, J. Xiao, Palladium-Catalysed Synthesis of Biaryl Phosphines. Tetrahedron 2004, 60, 4159–4168.
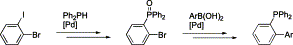
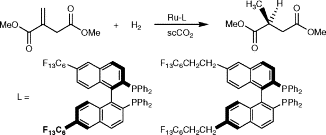
56. Y. Hu, D. J. Birdsall, et al. Ruthenium-Catalysed Asymmetric Hydrogenation with Fluoroalkylated BINAP Ligands in Supercritical CO2. J. Mol. Catal. Chem. 2004, 219, 57–60.
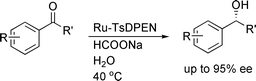
55. X. Wu, X. Li, et al. Accelerated Asymmetric Transfer Hydrogenation of Aromatic Ketones in Water. Org. Biomol. Chem. 2004, 2, 1818-1821.
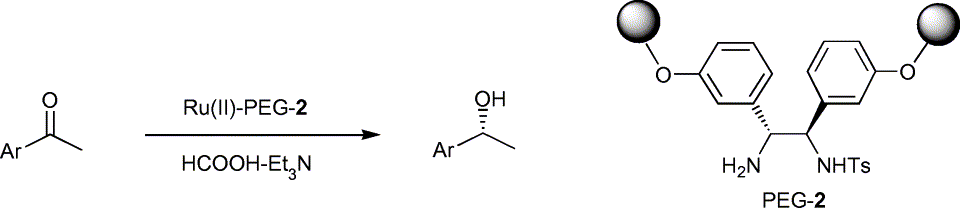
54. X. Li, W. Chen, et al. Asymmetric Transfer Hydrogenation of Ketones with a Polymer-Supported Chiral Diamine. Tetrahedron Lett. 2004, 45, 951–953.
53. L. Xu, J. Mo, et al. Palladium-Catalyzed Coupling Reactions of Bromo-Substituted Phenylphosphine Oxides: A Facile Route to Functionalized Arylphosphine Ligands. J. Organometal. Chem. 2003, 687, 301-312.
52. X. Li, W. Chen, et al. Asymmetric Hydrogenation of Ketones with Polymer-Supported Chiral 1,2-Diphenylethylenediamine. Org. Lett. 2003, 5, 4559–4561.
51. X. Li, X. You, et al. Some Insights into the Preparation of Pt/γ-Al2O3 Catalysts for the Enantioselective Hydrogenation of α-Ketoesters. Top. Catal. 2003, 25, 63-70.
50. J. Ross, J. Xiao, The Effect of Hydrogen Bonding on Allylic Alkylation and Isomerization Reactions in Ionic Liquids. Chem. Eur. J. 2003, 9, 4900-4906.
49. S. Subongkoj, S. Lange, et al. Effect of Diphosphine Ligands on Ruthenium Catalysed Asymmetric Hydrogenation of Ketones. J. Mol. Catal. Chem. 2003, 196, 125–129.
48. D. J. Adams, W. Chen, et al. Asymmetric Hydrogenation with Perfluoroalkylated Monodentate Phosphorus(III) Ligands in Supercritical CO2 and CH2Cl2. Green. Chem. 2003, 5, 118-122.
47. C. Baillie, J. Xiao, Catalytic Synthesis of Phosphines and Related Compounds. Curr. Org. Chem. 2003, 7, 477-514.
46. P. G. Jessop, R. A. Brown, et al. Pressure-Dependent Enantioselectivity in the Organozinc Addition to Aldehydes in Supercritical Fluids. J. Supercrit. Fluids. 2002, 24, 161–172.
45. L. Xu, W. Chen, J. Xiao, Palladium Catalysed Regioselective Arylation of Electron-Rich Olefins by Aryl Halides. J. Mol. Catal. Chem. 2002, 187, 189–193.

44. W. Chen, L. Xu, et al. New Approaches to Fluorinated Ligands and Their Application in Catalysis. Tetrahedron 2002, 58, 3889-3899.
43. J. Ross, J. Xiao, Friedel–Crafts Acylation Eeactions Using Metal Triflates in Ionic Liquid. Green Chem. 2002, 4, 129-133.
42. Y. Hu, W. Chen, et al. Fast and Unprecedented Chemoselective Hydroformylation of Acrylates with a Fluoropolymer Ligand in Supercritical CO2. Chem. Commun. 2002, 788-789.
41. C. Baillie, W. Chen, J. Xiao, Synthesis of Biphenyl-Based Phosphines by Suzuki Coupling. Tetrahedron Lett. 2001, 42, 9085–9088.
40. W. Chen, J. Xiao, Asymmetric Activation of Conformationally Flexible Monodentate Phosphites for Enantioselective Hydrogenation. Tetrahedron Lett. 2001, 42, 8737–8740.
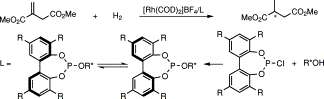
39. D. J. Birdsall, E. G. Hope, et al. Synthesis of Fluoroalkyl-Derivatised BINAP Ligands. Tetrahedron Lett. 2001, 42, 8551–8553.
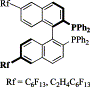
38. Y. Hu, W. Chen, et al. Carbonylated Phosphines as Ligands for Catalysis in Supercritical CO2. Organometallics 2001, 20, 3206–3208.
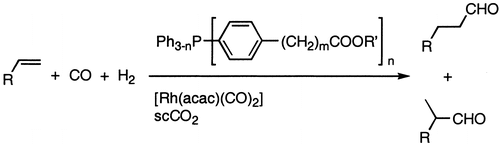
37. W. Chen, L. Xu, J. Xiao, A general Method to Fluorous Ponytail-Substituted Aromatics. Tetrahedron Lett. 2001, 42, 4275–4278.
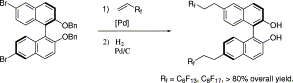
36. Y. Hu, W. Chen, et al. Rapid Hydroformylation of Alkyl Acrylates in Supercritical CO2. Chem. Commun. 2001, 725-726.
35. W. Chen, J. Xiao, Enantioselective Hydrogenation with Inexpensive, Easily Available Monodentate Phosphite Ligands. Tetrahedron Lett. 2001, 42, 2897–2899.
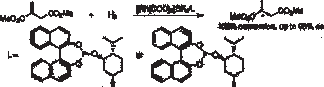
34. L. Xu, W. Chen, et al. Palladium-Catalyzed Regioselective Arylation of an Electron-Rich Olefin by Aryl Halides in Ionic Liquids. Org. Lett. 2001, 3, 295–297.
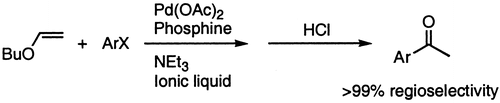
33. J. Ross, W. Chen, et al. Ligand Effects in Palladium-Catalyzed Allylic Alkylation in Ionic Liquids. Organometallics 2001, 20, 138–142.
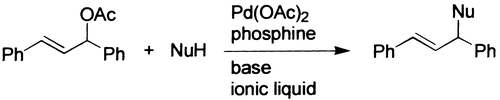
32. A. M. B. Osuna, W. Chen, et al. Effects of the Ponytails of Arylphosphines on the Hydroformylation of Higher Olefins in Supercritical CO2. J. Chem. Soc. Dalton Trans. 2000, 4052-4055.
31. W. Chen, L. Xu, J. Xiao, Palladium-Catalyzed Synthesis of Aqueous, Fluorous, and Supercritical CO2-Soluble Phosphines. Org. Lett. 2000, 2, 2675–2677.
![]()
30. W. Chen, J. Xiao, Novel and Efficient Synthesis of Perfluoroalkylated Arylphosphines. Tetrahedron Lett. 2000, 41, 3697–3700.
29. W. Chen, L. Xu, J. Xiao, Fluorous Soluble Polymercatalysts for the Fluorous Biphase Hydroformylation of Olefins. Chem. Commun. 2000, 839-840.
28. L. Xu, W. Chen, et al. Fluoroalkylated N-Heterocyclic Carbene Complexes of Palladium. J. Organometal. Chem. 2000, 598, 409–416.
27. L. Xu, W. Chen, J. Xiao, Heck Reaction in Ionic Liquids and the in Situ Identification of N-Heterocyclic Carbene Complexes of Palladium. Organometallics 2000, 19, 1123–1127.
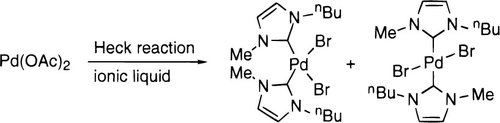
26. W. Chen, L. Xu, et al. Palladium Catalyzed Allylation Reactions in Ionic Liquids. Chem. Commun. 1999, 1247-1248.
25. L. Hao, J. Xiao, et al. Models for Platinum−Rhenium Bimetallic Catalysts: Sulfidation of Pt3Re Clusters. Organometallics 1997, 16, 2165–2174.
24. J. Xiao, S. C. A. Nefkens, et al. Asymmetric Hydrogenation of α,β-Unsaturated Carboxylic Acids in Supercritical Carbon Dioxide. Tetrahedron Lett. 1996, 37, 2813-2816.
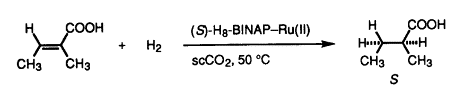
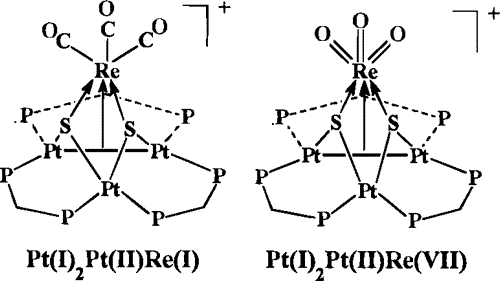
23. R. J. Puddephatt, J. Xiao, Coordinatively Unsaturated Pt and Pt-Re Clusters as Models for Surfaces and Bimetallic Catalysts. Math. Phys. Sci. 1996, 474, 407-435.
22. L. Hao, J. Xiao, et al. Platinum-Rhenium-Mercury and Related Cluster Chemistry. Inorg. Chem. 1996, 35, 658–666.
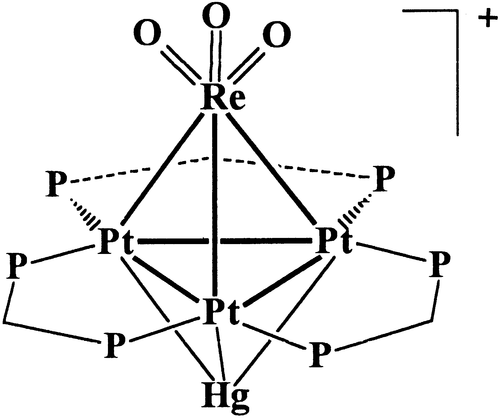
21. R. J. Puddephatt and J. Xiao, Met. Clusters Chem. 1996, 2, 605.
20. J. Xiao, R. J. Puddephatt, Pt-Re Clusters and Bimetallic Catalysts. Coord. Chem. Rev. 1995, 143, 457-500.
19. J. Xiao, L. Hao, R. J. Puddephatt, Models for Bimetallic Catalysis: Selectivity in Ligand Addition to a Coordinatively Unsaturated Pt3Re Cluster Cation. Organometallics 1995, 14, 4183—4193.
18. L. Hao, J. J. Vittal, et al. Pt5Re Cluster that Models a Bimetallic Catalyst: Evidence for PtRe Multiple Bonding. J. Am. Chem. Soc. 1995, 117, 8035-8036.
17. L. Hao, G. J. Spivak, et al. First Octahedral Platinum Cluster: Structure as a Function of Electron Count in Pt6 Clusters. J. Am. Chem. Soc. 1995, 117, 7011–7012.
16. J. Xiao, L. Hao, R. J. Puddephatt, Models for Bimetallic Catalysts: Anion Additions to Pt3Re Cluster Cations. Organometallics 1995, 14, 2194–2201.
15. J. Xiao, E. Kristof. et al. The coordinatively unsaturated cluster cations [Pt3{M(CO)3}(μ-Ph2PCH2PPh2)3]+ (M = Re, Mn). J. Organometal. Chem. 1995, 490, 1-6.
14. L. Hao, J. Xiao, et al. Models for Bimetallic Catalysts: Multiple Oxidation States in Pt3Re Cluster Cations. Angew. Chem. Int. Ed. 1995, 34, 346-348.
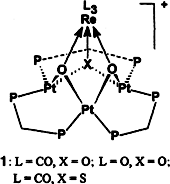
13. J. Xiao, L. Hao, et al. Models for Oxide Interactions in Bimetallic Catalysts: Oxo Clusters by Stepwise Oxidation of a Pt3Re Cluster. J. Am. Chem. Soc. 1995, 117, 6316–6326.
12. J. Xiao, L. Hao, et al. Models for Bimetallic Catalysts: Selectivity in Ligand Addition as a Function of Rhenium Oxidation State in Pt3Re Clusters. J. Chem. Soc. Chem. Commun. 1994, 2221-2222
11.L. Hao, J. Xiao, et al. Clusters as Models for Surface Catalysis: A Model for Sulfide Rffects on Pt–Re Catalysts. J. Chem. Soc. Chem. Commun. 1994, 2183-2184.
10. J. Xiao, R. J. Puddephatt, Models for Surface Catalysis: A Remarkable PtsCReOa) Cluster Cation. J. Am. Chem. Soc. 1994, 116, 1129-1130.
9. L. Manojlovic-Muir, K. W. Muir, et al. Syntheses and Structures of trans-[ReX(CO)(Ph2PCH2PPh2)2] (X = Cl, OReO3), including a Rhenium(I)-rhenium(VII) Complex. J. Organometal. Chem. 1993, 462, 235-241.
8. J. Xiao, J. J. Vittal, R. J. Puddephatt, The Addition of Oxygen to a Pt3Re Cluster Complex: A Model for Dissociative Chemisorption of Oxygen. J. Am. Chem. Soc. 1993, 115, 7882–7883.
7. J. Xiao, M. Cowie, "A-Frame" vs. "Open-Book" Geometries in Binuclear Complexes Bridged by Diphosphines and Mercaptothiazolinate Ligands. Unusual Examples Involving a Bridging Bis(diphenylphosphino)ethane Group. Can. J. Chem. 1993, 71, 726-737.
6. J. Xiao, B. D. Santarsiero, et al. Structure and Reactivity Studies of the Unusual Peroxo-Bridged Complex, [Ir2I2(CO)2(m-O2)(Ph2PCH2PPh2)2]: The First Compound Having a Peroxide Moiety Bridging a Metal-Metal Bond. J. Am. Chem. Soc. 1993, 115, 3212-3220.
5. J. Xiao, J. J. Vittal, R. J. Puddephatt, The Mechanism of Synthesis and the Unexpected Structure of the Cluster [Pt2Re2(µ-CO)2(CO)6(µ-dppm)2](dppm = Ph2PCH2PPh2). J. Chem. Soc. Chem. Commun. 1993, 167-169.
4. J. Xiao, M. Cowie, Alkyne-to-Vinylidene Tautomerism Mediated by Two Adjacent Metal Centers. Structures of Iridium Complexes [Ir2I2(CO)2(µ-CCHR)(Ph2PCH2PPh2)2] (R = H, Ph). Organometallics 1993, 12, 463–472.
3. B. A. Vaartstra, J. Xiao, et al. Binding of π-Acid Ligands in Diiridium and Rhodium-Iridium Iodo Complexes, including Rare Examples of Ethylene Coordination in "A-Frame" Compounds. Structure of [Ir2I2(CO)(μ-CO)(Ph2PCH2PPh2)2]CH2Cl2. Organometallics 1991,10, 2708-2717.
2. B. A. Vaartstra, J. Xiao, M. Cowie, First Example of a Peroxo-Bridged Complex Having an Accompanying Metal-Metal Bond. J. Am. Chem. Soc. 1990, 112, 9425–9426.
1. Effects of Rhenium, Tin and Iridium on Platinum Catalyst in Reforming of Light Hydrocarbons. J. Wang, J. Xiao, J. Lin, Shiyou Xuebao, Shiyou Jiagong 1989, 5, 61-70.
19. X. Wu, J. Xiao, “Reduction of C=O to CHOH by Metal-Catalysed Hydrogenation and Transfer Hydrogenation” in Comprehensive Organic Synthesis, J. Clayden, G. Molander, and P. Knochel(Eds.), Elsevier, 2014.
18. S. Liu, J. Xiao, in Bridging Heterogeneous and Homogeneous Catalysis, C. Li and Y. Liu (Eds.), Wiley-VCH, 2014.
17. C. Wang, J. Xiao, in Stereoselective Formation of Amines, W. Li and X. Zhang (Eds.), Springer, 2014.
16. X. Wu, J. Xiao, “Hydrogenation and Transfer Hydrogenation in Water”, In Metal Catalysis in Water, P. H. Dixneuf and V. Cadierno (Eds.), Wiley-VCH, 2014.
15. S. Liu, J. Xiao, “Heck Reaction of Electron-rich Alkenes with one EDG: Aryl-X and Hetaryl-X Electrophiles”, In Science of Synthesis: Cross-coupling and Heck-Type Reactions, M. Larhed, G. A. Molander and J. P. Wolfe (Eds.), Thieme, 2013.
14. X. Wu, J. Xiao, “Reduction of Carbonyl and Imino Groups”, In Science of Synthesis: Water in Organic Synthesis, S. Kobayashi (Ed.), Thieme, 2012.
13. L. Xu, X. Wu, J. Xiao, “Stereoselective Reduction of Imino Groups”, In Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups, G. A. Molander (Ed.), Georg Thieme Verlag KG, 2010.
12. X. Wu, J. Xiao, “Green Reduction in Water”, in Green Solvents, Handbook of Green Chemistry, P.T. Anastas, C.J. Li (Eds.), Wiley-VCH, 2010.
11. L. Xu, J. Xiao, “Asymmetric Catalysis in Ionic Liquid”, in Recoverable and Recyclable Catalysts, M. Benaglia (Ed.), Wiley-VCH, 2009.
10. J. Mo, L. Xu, J. Xiao, “Ionic Liquid-Promoted Regioselective Catalysis by Palladium”, in Proceedings of the 14th International Symposium on Molten Salts, R. A. Mantz, (Ed.),The Electrochemical Society, 2004.
9. J. Xiao, “Environmental Catalysis in Organic Synthesis”, in Environmental Catalysis, V. Grassian, (Ed.), Marcel Dekker, 2004.
8. C. Baillie, L. Xu, J. Xiao, “Synthesis of Phosphines by C-C Coupling Chemistry”, in Catalysts in Fine Chemical Synthesis, S. Roberts, J. Xiao, etc. (Eds.), Wiley, 2004.
7. J. Mo, L. Xu, J. Xiao, “Ionic Liquid-Promoted α-Arylation of Vinyl Ethers”, in Catalysts in Fine Chemical Synthesis, S. Roberts, J. Xiao, etc. (Eds.), Wiley, 2004.
6. W. Chen, J. Xiao, “Synthesis of Perfluoroalkyl Aryl Phosphines by Copper-Mediated Cross Coupling”, in Handbook of Fluorous Chemistry, J. A. Gladysz, D. P. Curran, I.T. Horvath, (Eds.), Wiley-VCH, 2004.
5. W. Chen, J. Xiao, “Synthesis of Fluoroalkyl Arylphosphines by the Heck Reaction”, in Handbook of Fluorous Chemistry, J. A. Gladysz, D. P. Curran, I. T. Horvath, (Eds.), Wiley-VCH, 2004.
4. J. Ross, J. Xiao, “The Importance of Hydrogen Bonding to Catalysis in Ionic Liquids. Inhibition of Allylic Substitution and Isomerization [bmim][BF4]”, in Ionic Liquids as Green Solvents: Progress & Prospects, K. Seddon, R. Rogers, (Eds.), American Chemical Society, 2003.
3. L. Xu, A. Banet, J, Xiao, “From Designing Ligands to Designer Solvents: Homogeneous Catalysis Made Easy, Cheap and Green”, in CHEMPOR, F.R Ribeiro, J. J. C. Pinto, (Eds.), 2001.
2. W. Chen, A. M. Banet Osuna, J, Xiao, “Soluble Fluoropolymer Catalysts for Hydroformylation of Olefins in Fluorous Phases and Supercritical CO2”, in Supported Catalysts and Their Applications, D. C. Sherrington, A. P. Kybett (Eds.), Royal Society of Chemistry, 2001.
1. A. Banet, I. Chadbond, J. Xiao, “Supercritical Fluids: Chemistry and Materials”, M. Poliakoff, M. W. George, S. M. Howdle (Eds.), International Society for the Advancement of Supercritical Fluids, 1999