|
Note: the numbers
in square brackets refer (and link) to the List of
Publications
Light-Induced Charge Separation and Recombination Dynamics in
Proteins and Model Compounds
Reaction
Centres of Photosynthetic Bacteria and Photosystem
II of Plants
The focus of these investigations was the measurement
of the magnetic field dependent recombination dynamics of the light-induced
primary radical pair of photosynthetic reaction centres on the ns-time
scale. Various native as well as mutagenetically
and chemically altered reaction centres were investigated. On the basis of
high-precision measurements and extensive theoretical work, the observed
radical pair recombination dynamics could be understood in all details. [6]
Using these results, the free energies as well as electronic couplings of
all relevant states were determined. [2,
14]
These results form the basis of the discussion of the mechanism and high
efficiency of fast charge separation in photosynthetic reaction centres. [3,
5,
8]
A comparative investigation of bacterial reaction centres and Photosystem II of plants confirmed the high structural
similarity of the two proteins. [7]
Synthetic
Electron-Donor/Acceptor-Systems
Using transient absorption spectroscopy, electron
transfer was shown to be the fluorescence quenching process in a series of
bridged donor/acceptor-systems. The dynamics of the charge recombination
process was determined on the ns-time scale [1]
DNA-Photolyase
Results on the enzyme DNA-photolyase,
which repairs UV-induced pyrimidine dimers on the DNA using light-induced electron
transfer, allowed the assignment of a previously observed transient
absorption signal at 400 nm and a better estimate of the dynamics of
the associated state. [9]
Vibronic Bands as Highly Sensitive Sensors for
Charge Transfer Reactions
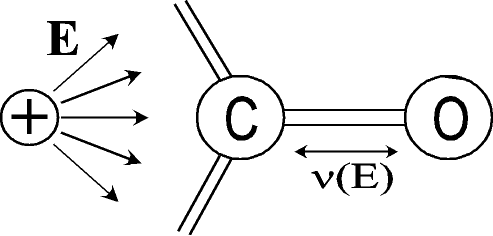 In many cases,
electronic transitions of the intermediate states of small molecules are hard
to observe in optical spectroscopy. In these cases, charge transfer and
dissociation reactions can be followed by time-resolved IR spectroscopy via
their effect on the vibrational frequency of
suitable bonds. The "sensor" bond does not need to be part of the
molecule under investigation, but may experience vibrational
shifts due to electrostatic effects on its vibrational
frequency (vibrational Stark effect). The
feasibility of this approach for following charge transfer reactions on the
fs-time scale was tested on specifically designed
molecules, which consist of ferrocene with an
attached carbonyl group as charge sensor. [18] In many cases,
electronic transitions of the intermediate states of small molecules are hard
to observe in optical spectroscopy. In these cases, charge transfer and
dissociation reactions can be followed by time-resolved IR spectroscopy via
their effect on the vibrational frequency of
suitable bonds. The "sensor" bond does not need to be part of the
molecule under investigation, but may experience vibrational
shifts due to electrostatic effects on its vibrational
frequency (vibrational Stark effect). The
feasibility of this approach for following charge transfer reactions on the
fs-time scale was tested on specifically designed
molecules, which consist of ferrocene with an
attached carbonyl group as charge sensor. [18]
Light-Induced
Relaxation Processes in Condensed Phase
Energy Relaxation in Proteins
Transient absorption measurements with fs-time resolution on myoglobin allowed the direct
observation of electronic and vibronic relaxation
after excitation of the heme cofactor. Vibronic
energy was observed to be transfered from heme to
protein in a few picoseconds. [19]
Ligand
Dynamics in Heme Proteins
The goal of these investigations was a detailed
understanding of the recombination of NO in myoglobin, which proceeds on
the time scale of 100 ps. For this purpose, the dynamics of NO
recombination in myoglobin from different species was measured. The
observed differences can be related to differences in the primary sequence.
Furthermore, we compared the NO recombination dynamics after excitation of
heme to different electronic states (different excess energy). These
allowed to conclude on fast intramolecular
energy dissipation before ligand dissociation. [Book7]
Folding Dynamics of Small Peptides
Using specifically designed de novo peptides,
in which the formation of secondary structure can be induced by a fast photodissociative trigger, the folding dynamics of a-helices was
investigated with a time resolution of 100 fs.
It was shown that no folding occurs before 100 ps. [12,
13]
The rate constant for the recombination of radicals at the peptide ends,
formed during the photochemical triggering, was observed to have an unusual
1/t-time dependence in the whole range investigated, i.e. between 1 ps and 10 ms. [16]
Coherent Motion after Bond Dissociation in
Small Molecules
Measurements on various small molecules in solution
were performed to observe coherent motions after bond dissociation. Here we
focused on the observation of rotational motion, which can be well traced
by measuring the anisotropy of transient absorption.
Mercury
Iodide
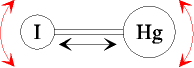 The orientational relaxation of HgI,
formed by photodissociation of HgI2,
was found to correspond to the behaviour of a free rotor in the first
500 fs. After that time collisions with
solvent molecules induce diffusive rotational motion. Of particular
interest was the observation of an oscillating rate of anisotropy decay,
with the oscillations paralleling the previously observed coherent
vibration of HgI after its formation by a short
laser pulse. The oscillating rate of anisotropy
decay can be well understood by the variation of the moment of inertia
during the coherent vibration. This constitutes the first observation of a vibronic wave packet via the rotational dynamics of a
molecule. [10,
11] The orientational relaxation of HgI,
formed by photodissociation of HgI2,
was found to correspond to the behaviour of a free rotor in the first
500 fs. After that time collisions with
solvent molecules induce diffusive rotational motion. Of particular
interest was the observation of an oscillating rate of anisotropy decay,
with the oscillations paralleling the previously observed coherent
vibration of HgI after its formation by a short
laser pulse. The oscillating rate of anisotropy
decay can be well understood by the variation of the moment of inertia
during the coherent vibration. This constitutes the first observation of a vibronic wave packet via the rotational dynamics of a
molecule. [10,
11]
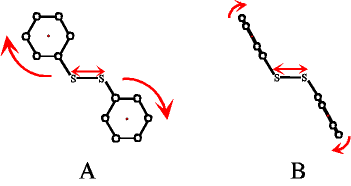
Aryl Disullfides
The anisotropy of transient thiyl radical absorption
after photodissociation of the disulfide bond in
various substituted aryl disulfides was observed. Depending on the initial
structure of the aryl disulfide, the orientational
motion was found to be dominated by rotation around the phenyl ring axis
(if the rings are parallel to the disulfide bond, A) or by a tumbling
motion of the rings in the solvent cage (if the rings are orthogonal to the
disulfide bond, B). In the latter case, the time-dependent anisotropy data
show oscillations, which are indicative of a coherent libration
of the phenyl rings in their solvent cage. [15]
Structural Properties of Polyamino
Acid Catalysts
In collaboration with the group of Prof. S.M. Roberts
at the University of Liverpool, we studied polyamino
acids which catalyse the asymmetric epoxidation
of a wide range of alkenes. Secondary structure information for these
catalysts was obtained using FTIR spectroscopy, and it was shown that the
catalytically active components of these polymers have a-helical
structure. [20]
|