Melanopark
Home --- Melanoma Cell Lines ---LRRK2 cell lines and lentivirus---Plasmids
Glycerol Stocks ---Antibodies --- Inhibitors and Drugs ---Primers --- siRNA Oligos
Parkinson’s disease (PD) is a progressive movement disorder affecting about 1.8% of people over the age of 65, characterized by the degeneration of the midbrain dopaminergic neurons in the substantia nigra. It is predominantly a sporadic disease, but about 10% of cases can be attributed to Mendelian inheritance. Neurodegenerative diseases are often associated with decreased cancer risk. However PD patients have an increased risk of melanoma and it has been proposed that the two conditions may share some biological pathways.
LRRK2 is a multi-domain protein, which uniquely possesses both protein kinase and GTPase catalytic activities (ROC-COR domains). Autosomal dominant mutations of LRRK2 represent one of the principal genetic risk factors for Parkinson’s Disease (PD), which results in preferential loss of neuromelanin containing, dopaminergic neurons from the substantia nigra. The (patho)physiological roles of LRRK2 are currently unclear. However, it has been linked to the regulation of endolysosmal membrane trafficking through complex formation with other PD-related gene-products (Rab7L1 and Vps35). LRRK2 is also highly expressed in melanoma cells (that derive from skin melanocytes). Melanocytes specifically produce melanin within the lysosome-related organelles known as melanosomes, in a process referred to as melanogenesis.
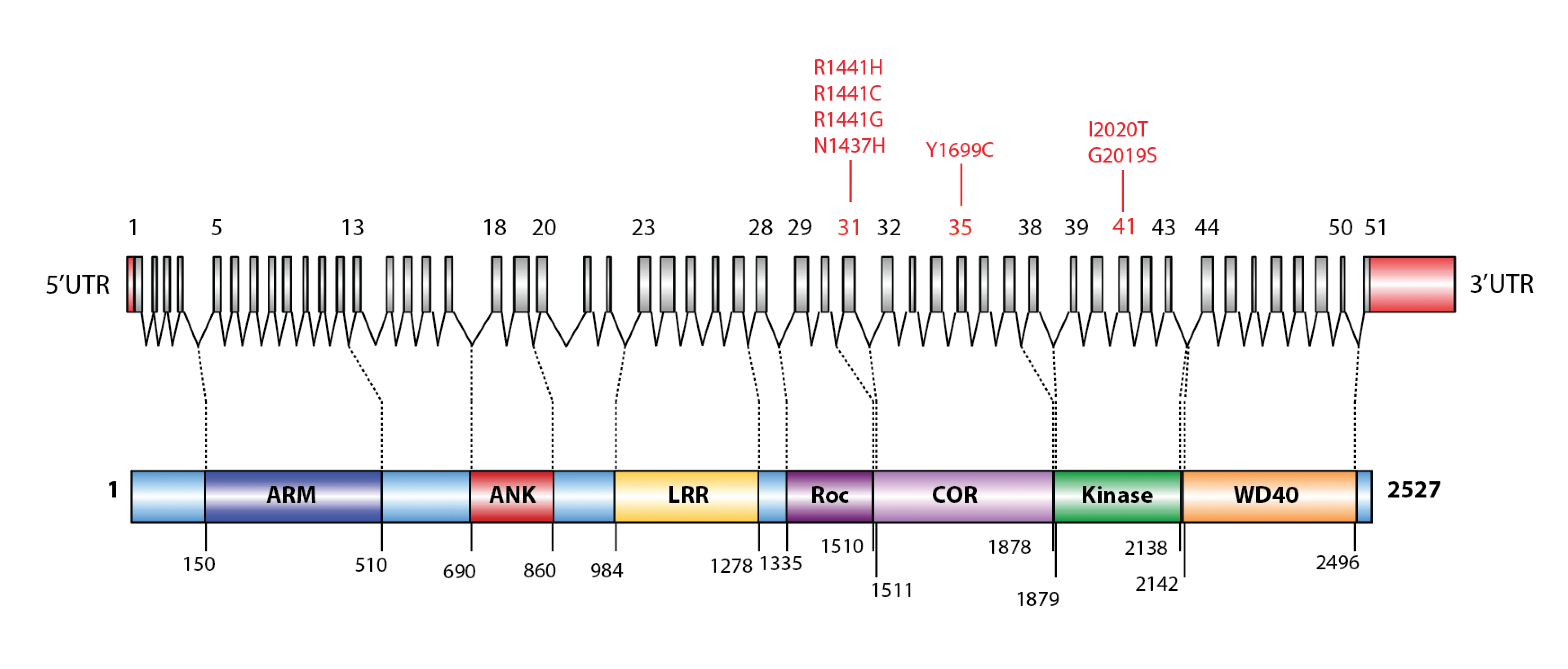
Figure 1. Schematic representation of the LRRK2 protein, indicating some established pathogenic mutations (with correspondence between exons and protein domains).
ARM (Armadillo), ANK (ankyrin repeat), LRR (leucine-rich repeat), Roc (Ras of complex proteins: GTPase), COR (COOH terminal of Roc), kinase domain, and WD40. Common PD-associated mutations are indicated in red (Figure adapted from Corti et al., 2011).
Recently, it has been shown that LRRK2 regulates (inhibition) a subset of Rab GTPases. LRRK2 phosphorylates the GDP bound form of Rabs to maintain them in an inactive state.
Interestingly, amoe.
Interestingly, among those Rabs we can find several RAbs that have been implicated in melanosomes positioning and/ or maturation: Rab1A, Rab3A, Rab7A, Rab8A and Rab27A for positioning; Rab7A and Rab9A for melanosmes maturation. Rab7L1 that inuration. Rab7L1 that interacts with LRRK2 is also a substrate but has not been shown to be important for melanogenesis. On the contrary, Rab32 and Rab38 (that are very close to Rab7L1) are not LRRK2 substrates, but are implicated in melanogenesis (traffic of melanogenic enzymes).
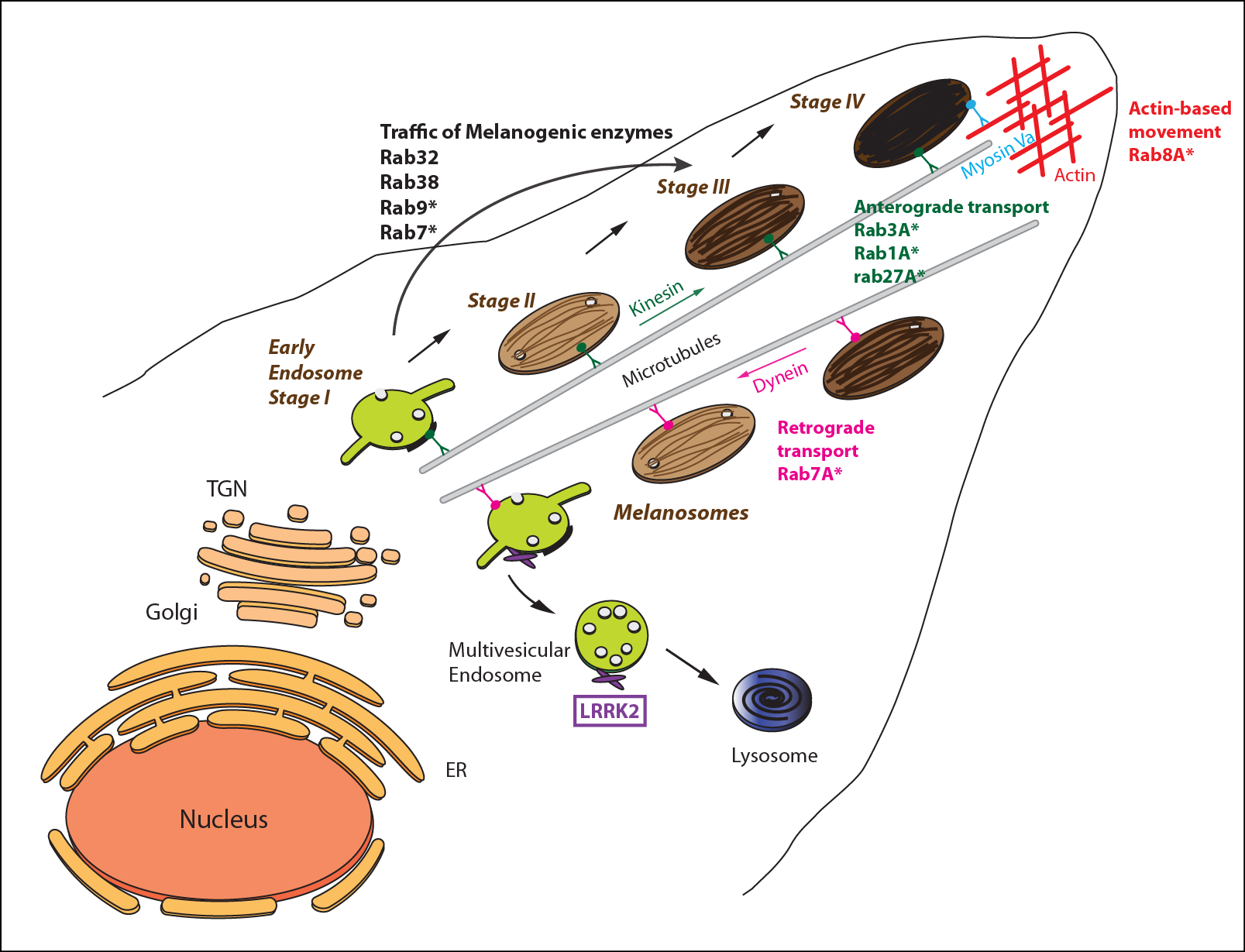
Figure 2. Schematic representation of the melanogenesis pathway and melanosomes movement in skin melanocyte.
Melanosomes derive from the endosomal system, stage I correspond to a sorting endosome characterized by the presence of few intraluminal vesicles and clathrin coats. The stage II melanosome is characterized by the presence of intraluminal fibrils that start to form at stage I and serve as a scaffold for melanin deposition. The stage III melanosome is characterized by the beginning of melanin synthesis and deposition upon the intraluminal fibrils. The traffic of enzymes implicated in the synthesis of the melanin pigment involves Rab GTPases such as Rab7, Rab9, Rab32 and Rab38. Stage IV is a fully pigmented compartment in which the fibrils are no longer visible. Melanosomes are transported along both microtubules and the cortical actin network in the tips of the melanocytes, while progressing from an early stage I to a
mature stage IV melanosome. The anterograde microtubule-dependent transport is controlled by kinesin motors (transport to the tips) whereas the retrograde transport is controlled by the dynein motor (transport toward the nucleus). At the tips of the melanocyte, the actin-based movement of melanosomes is controlled by the myosin Va motor. The transport and movement of melanosomes involve differents Rab GTPases: Rab3A, Rab1A and Rab27Aab27A for the anterograde transport; Rab7A for the retrograde transport and Rab8A for the actin-based movement.
LRRK2 may localized to endosomes and melanosomes membranes and mayd may and interact with RAb32 and Rab38. LRRK2 may also phosphorylate some Rab GTPases that are involved in melanosomes transport, movement and maturation (asterisks indicate the Rabs that have been shown to be phosphorylated by LRRK2).
This project aims to examine the substrates and function of LRRK2 in pigmented melanoma cells.
Copyright 2016© - Site designed by Leila Rochin
