Cell Signalling Laboratory
| |
| Home |
| Selected publications |
| Group Members |
| Links |
| Vacancies |
| RAS PROTEINS |
Ras proteins are molecular switches that exist in a GDP-bound inactive state or GTP-bound active state. Accessory proteins switch Ras between these two states:
Guanine nucleotide exchange factors (GEFs): dislodge GDP which is then replaced by free GTP from the cytosol. GTP is present at 10x the concentration as GDP in the cytosol meaning that GEFs activity usually results in activation of a Ras molecule. At least 18 GEFs have been characterised, the most studied is Sos.
GTPase activating proteins (GAPs): stimulate the intrinsic GTPase activity of Ras enabling GTP to be hydrolysed to GDP. Ras is therefore switched off in a few milliseconds instead of minutes. At least 14 GAPs have been characterised, the best known is p120Ras GAP.
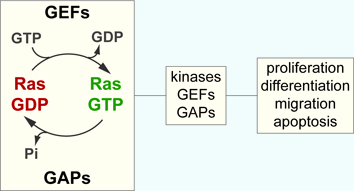
Signalling: When Ras is GTP bound it causes a change in its shape revealing a binding site enabling interaction with many downstream signalling proteins. Recruitment of these proteins to the membrane enables them to become activated by other proteins already present such as kinases or phosphatases. Some of the best characterised effectors are Raf and phosphatidylinositol 3-kinase (PI3K).
Function: Ras controls many signalling
pathways. Generally these promote cell division and inhibit cell death.
In cancer when Ras is mutated, these pathways are hyperactivated causing unregulated cell proliferation.
Latest
news Group members
. |