Current research themes
Regulation of epithelial cell renewal in the GI tract
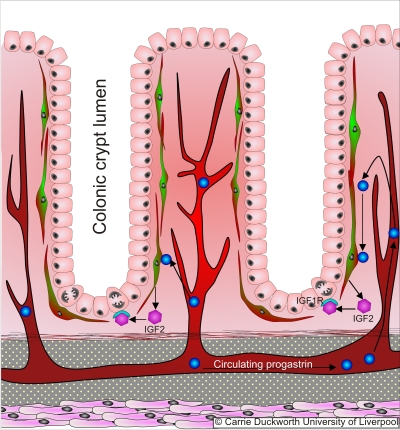
|
The epithelial layer of the intestine is continuously renewed with a rapid cell turnover. This tighly regulated process is maintained by stem cell division at the base of colonic crypts, migration and differentiation of daughter cells along the crypt axis, programmed cell death and shedding of cells into the gut lumen. In our lab, we investigate how the dysregulation of these processes contributes to the development of gastrointestinal disease.Over recent years, we have identified a novel regulator of cell differentiation in the colon. Bak is a pro-apoptotic member of the bcl-2 family which exerts its classical function by moduating mitochondrial outer memberane permeability. Using transgenic mice with a germline deletion of the bak gene, we were able to determine that this gene along with modulating the apoptotic response to radiation and chemically-induced apoptosis, suppresses colonic goblet cell differentiation and promotes enteroendocrine cell formation (Duckworth et al Gastroenterology 2009). We are currently determining whether bak has a similar function in the stomach.Intestinal epithelial cell dynamics are also modulated by their interaction with peri-cryptal myofibroblasts. Proteins that circulate in the blood stream are able to interact with these myofibroblasts to release factors that act on epithelial stem and transit amplifying cells to modulate the rate of cell division. Progastrin, a protein secreted into the circulation in high abundance by colon cancers, is able to stimulate insulin-like growth factor 2 (IGF-2) release from myofibroblasts that can stimulate excessive proliferation of the intestinal epithelium. |
Inflammatory bowel disease (IBD) and IBD-associated colon cancer
NF-kB signalling pathways play important roles in the pathogenesis of inflammatory bowel disease (IBD) and IBD-related colon cancers. Five members of the NF-kB family of transcription factors (p50 (NF-kB1), p65 (RelA), p52 (NF-kB2), RelB, c-Rel) signal via classical or alternative activation pathways to modulate pro-death or pro-survival responses to cellular stresses (e.g. infection, inflammation). Several effective therapies for IBD, including steroids, affect NF-kB signalling and colonic expression levels of p65 are elevated in patients with IBD (Schreiber et al 1998). The majority of studies to date have focused on classical pathway signalling (Greten et al 2004, Nenci et al 2007, Steinbrecher et al 2008) however, accumulating evidence suggests that the alternative NF-kB activation pathway also plays important roles. For instance, perturbed regulation of alternative pathway signalling modulates intestinal responses to the helminth Trichuris muris (Artis et al 2002). Very few studies to date have been conducted to assess the roles of alternative pathway signalling during the active, recovery and quiescent stages of IBD in mouse or man. Recent studies in our laboratory have however demonstrated almost complete resistance to dextran sulphate sodium (DSS)-induced colitis in mice deficient in NF-kB2. We are now
