
Biocatalysis and Chemoenzymatic Synthesis
The use of enzymes to catalyse highly efficient and selective reactions is well established. We have an ongoing interest in developing new biotransformations for application in organic sysnthesis. In particular, enzyme reactions that give a definite advantage over or are complementary to methodologies available using ‘conventional’ chemistry. Chiral enol esters are useful synthetic intermediates and can be converted into enolates, oxidatively rearranged to α,β-unsturated enones or oxidatively cleaved. We developed the enzyme kinetic resolution of racemic enol esters derived from prochiral ketones.[1] This gives enolate equivalents that are difficult to obtain by other methods such as the use of specialised chiral lithium amides. The product from the kinetic resolution is the symmetrical ketone 1 which can be recycled, thus leading to greater than 50% yields of the unreacted chiral enol ester (S)-(+)-2 .[2]
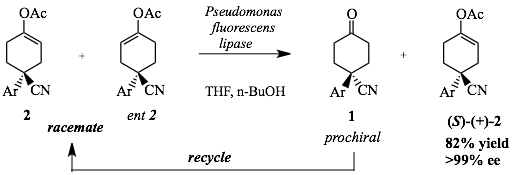
Figure 1: Enzyme kinetic resolution of racemic enol ester 2 derived from prochiral ketone 1
This was used for the synthesis of a tachykinin NK2 antagonists that was being developed at Pfizer for the treatment of neuroinflammatory conditions (Figure 2).[3]

Figure 2: The (S)-(+)-enol acetate 2 was oxidatively cleaved and in 6 steps converted to the
NK-2 antagonist 3.
We used a similar lipase biotransformation for the efficient resolution of [2.2.2] bicyclic diketones. These are used for the preparation of Hayashi’s chiral diene ligands and can otherwise only be accessed by chiral HPLC. Screening for solvent conditions and solid support optimisation for the resolution of (±)-enol acetate 4 lead to a highly selective transesterification in pentane using a modified hydrophobic silica support (GS-03) developed by PhosphonicS (E = 270). [4]
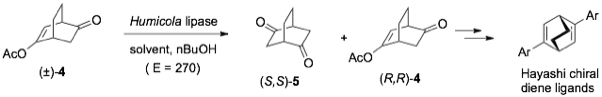
Figure 3: Accessing Hayashi’s diene ligands using highly selective lipase resolution.
We then developed a new synthetic route to a series of 1,4-disubstituted [2.2.2]diketones as chiral ligand precursors and a scalable biphasic resolution of the (±)-enol hexanoate 6 to give multigram quantities of enantiomerically pure (S,S)-6. This material provided a key building block for the rapid synthesis of a new range of [2.2.2] bicyclic ligands that show remarkable electronic tenability in rhodium-catalysed asymmetric conjugate addition reactions.[5]
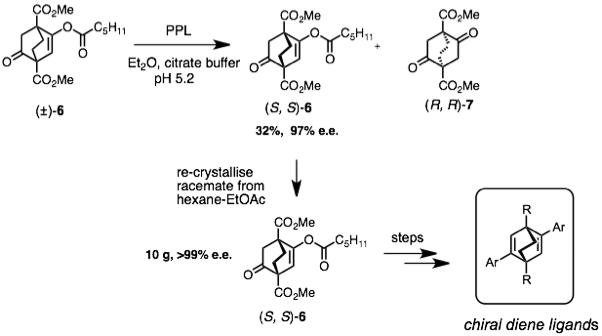
Figure 4: Biphasic bioresolution leads to 10g quantities of chiral diene precursor (S,S)-6.
(1) Carnell, A. In Journal of Molecular Catalysis B-Enzymatic; 2002; Vol. 19, pp. 83–92.
(2) Allan, G.; Carnell, A.; Kroutil, W. Tetrahedron Letters 2001, 42, 5959–5962.
(3) Allan, G.; Carnell, A.; Hernandez, M.; Pettman, A. Tetrahedron 2001, 57, 8193–8202.
(4) Luo, Y.; Carnell, A. J. J. Org. Chem. 2010, 75, 2057–2060.
(5) Luo, Y.; Carnell, A. J. Angew. Chem. Int. Ed. Engl. 2010, 49, 2750–2754.
