
Electronically Tunable Chiral Dienes for Asymmetric Catalysis
We are interested in the development and application of biotransformations in synthetic organic chemistry to provide more efficient and sustainable routes to key building blocks. As an example, chiral dienes have become attractive ligands in asymmetric catalysis in work pioneered by Hayashi, Carreira and others. Some of the dienes ligands or their precursors are difficult to access in homochiral form and require preparative chiral HPLC separations.
Enzyme resolution of enol ester (±)-1 gave access to 10g quantities of homochiral diketone diester (S,S)-2 which can be used to make 1,4-dimethyl substituted bicyclic [2.2.2] diene ligands. These were shown to be electronically tunable by choice of aryl group for asymmetric conjugate additions to different substrate types, delivering excellent activity and enantioselectivity.[1]
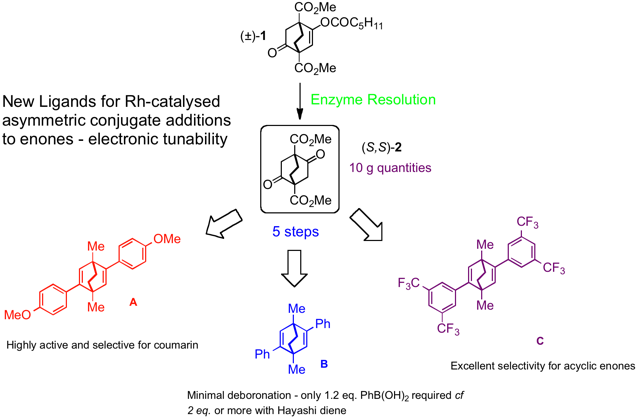
The electron rich ligand A gave a significant rate enhancement over other ligands (eg. B or Hayashi’s ligand) for addition to 6-methyl coumarin.

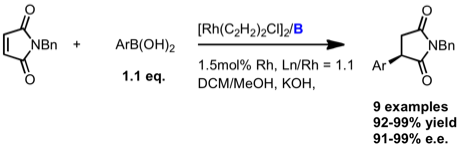
Ligand B gave excellent results for asymmetric conjugate addition to N-benzylmaleimide using just 1.1eq. of aryl boronic acid, compared to the normally required 2 eq. Other ligands generally give lower selectivity for this type of substrate and require more equivalents of aryl boronate.
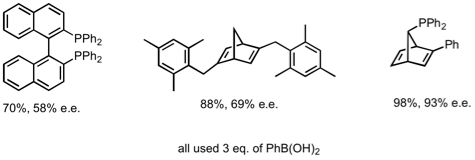
The electron poor ligand C gave excellent results for addition to acyclic enones. By comparison ligand B gave 52% ee.

We are currently exploring the correlation between electronic properties of these ligands and activity/selectivity.
(1) Luo, Y.; Carnell, A. J. Angew. Chem. Int. Ed. Engl. 2010, 49, 2750–2754;
Luo, Y.; Carnell, A. J. J. Org. Chem. 2010, 75, 2057–2060
