Project
Fast Events in Protein Folding - Further DetailsAmide I-band as Sensitive Sensor of Secondary Structure
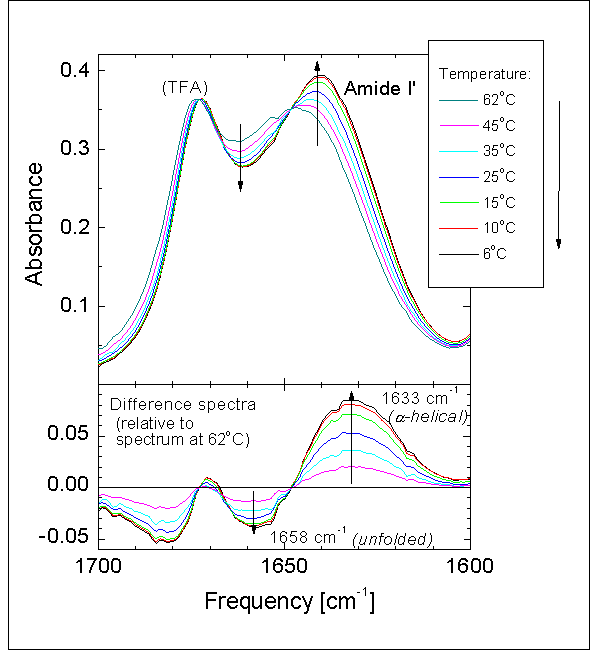
The amide I band of peptides and proteins, which mainly involves the carbonyl stretching vibrations of the peptide backbone, is a sensitive marker of peptide secondary structure, as the vibrational frequency of each C=O bond depends on hydrogen bonding and the interactions between the amide units, both of which are influenced by the secondary structure.
The temperature-dependent amide I spectra shown here are those of a particular de-novo peptide used for the investigation of fast folding processes (peptide 4 of the investigated peptides, but here without the constraining disulfide bond). This linear peptide is mainly a-helical, with the helical content decreasing upon raising the temperature. Unlike larger proteins, which "melt" in a concerted process at one well-defined transition temperature, short peptides show a gradual melting extending over 10s of degrees, with the helix slowly decreasing from the full peptide length at low temperatures to zero at high temperatures.
As the figure shows, this melting is accompanied by a shift of the amide I band from 1633 cm-1 to 1658 cm-1. The difference spectra with their well defined isosbestic point confirm that this shift is not a gradual shift of all of the amide I band (i.e. a "simple" temperature dependence due to interaction with the solvent), but rather corresponds to a population shift of a species absorbing at 1633 cm-1 (amino acid residues in a-helical conformation) to a species absorbing at 1658 cm-1 (AAR in the unfolded conformation).
When inducing a sudden temperature jump, the peptide will not immediately adopt the more unfolded conformation. Since the amide I band is sensitive to the secondary structure, time-resolved IR-absorbance measurements allow one to follow the dynamics of the unfolding reaction.
Back to the Project Description
Last update: 17.02.2000