Dr. Martin Volk
Surface Science Research Centre
University of Liverpool
Liverpool L69 3BX
United Kingdom
Tel. +44-151-794-3317
Fax +44-151-708-0662
Email
Project
Fast Events in Protein FoldingUnderstanding the detailed mechanism of protein folding is one of the most challenging questions currently investigated in biological research. In a highly efficient process an unfolded polypeptide adopts a well-defined three-dimensional structure, which defines the biological or catalytic function of the protein. The investigation of the dynamic aspects of this folding process is important for elucidating the folding mechanism of natural proteins. Moreover, it is of high relevance for predicting the structure of engineered peptides which are designed to offer new (e.g. catalytic) functions. Such peptides will adopt the anticipated functional structure only if there is an efficient folding pathway which avoids the accumulation of metastable non-functional folds.
The first phase of this folding process is generally believed to be the formation of secondary structural elements like a-helices, b-sheets or b-turns. These regions then act as nucleation sites for the further collapse to the native structure. Thus, secondary structure formation, which occurs on the time scale of nanoseconds to microseconds, is of utmost importance for protein folding. In spite of this importance, only a very limited number of investigations on the relevant time scale were performed so far (click here for more information on previous investigations).
The focus of this experimental project is the process of alpha-helix folding and unfolding. The samples will be short synthetic model peptides which form "perfect" a -helices, thus avoiding the heterogeneous mixture of structural elements found in natural proteins. The goal is to achieve a detailed understanding of the dynamics of a -helix formation and the factors governing it, including questions like:
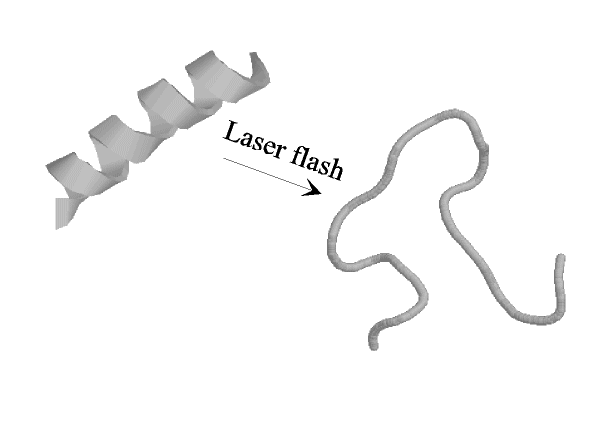
The experimental approach used is the induction of a fast temperature jump using a nanosecond laser flash, which heats the solvent to a temperature above the melting point of the a-helix. The dynamics of the ensuing unfolding are observed by time-resolved IR-spectroscopy of a vibrational band whose spectral position is highly sensitive to the secondary structure (the so-called amide I-band) and thus allows one to directly follow the folding/unfolding process.
By changing the primary sequence of the synthetic peptides it will be possible to systematically investigate internal factors governing protein folding, such as the interaction between specific amino acid residues or the length of the peptide. On the other hand, the observation of unfolding under different solvent conditions will enable us to elucidate external factors affecting the folding dynamics.
Further Reading:
Callender et al., Annu. Rev. Phys. Chem. 1998, 49, 173-202
Acc. Chem. Res. 1998, vol. 31, no. 11 (special issue on protein folding).
For more information, please send me an email or contact me under the above address.