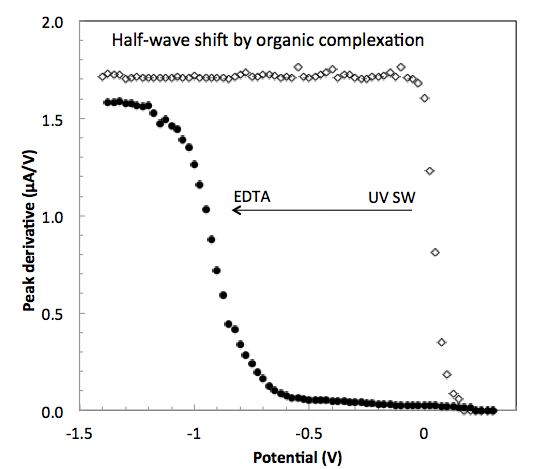
The reduction potential shifts negative when the oxidised species of a redox couple is stabilised by complexation. This shift can be used to determine the stability of complexes, known or unknown, in natural waters. The shift is apparent in a polarographic wave and also in a DC-voltammetric scan, if the metal species occur at sufficiently high concentration. Because of the low concentration of trace metals in natural waters, this requires a pre-concentration step which in anodic stripping voltammetry can be done by plating. By carrying out the plating step sequentially at a series of increasingly negative potentials, a polarographic wave is built up step by step. This can be done using the mercury drop and the mercury film electrode. However, copper is determined with better sensitivity using a gold microwire electrode. For this reason a pseudopolarographic method has been set up using the microwire electrode. The shift in the wave is small for reversible complexes, whereas it is large for irreversible species.
See the relevant references below.
Gibbon-Walsh, K., P. Salaün and C. M. G. Van Den Berg (2012). "Pseudopolarography of copper complexes in seawater using a vibrating gold microwire electrode." Journal of Physical Chemistry A 116(25): 6609-6620.
Bi, Z., P. Salaün and C. M. G. van den Berg (2013). "The speciation of lead in seawater by pseudopolarography using a vibrating silver amalgam microwire electrode." Marine Chemistry 151(0): 1-12.