Shedding new light on Pain pathways using
Jelly-fish genes.
Richard Morris and Richard Barrett-Jolley
Veterinary Neurobiology, University
of Liverpool
Home
page
Studies to further the development of
pain relief in humans and animals
Description of research
Aims
Recent developments in molecular
biology methods have permitted the incorporation of marker fluorescent proteins
into transgenic mice. One of the most commonly used marker proteins fluoresces
green (GFP) and was originally identified in a jellyfish. This fluorescent
protein permits labeling of cells on the basis of the genes they express. The
huge advantage of this approach is that these proteins can be seen in living
cells and do not alter the normal function of these cells. Thus for example, in
principle, it would be possible to produce a transgenic mouse in which all the
neurons that express m-opioid receptors
are bright green. This can then be used to target these cells for
electrophysiological recording, track cells in development and in cell culture
and to sort these cells for gene analysis. This technology is revolutionizing
neurobiology and as I will show below is already generating startling new
results.
Expressed
simply our overall aim is to start to exploit this new technology fully to
investigate nociceptive systems. This technology is quite new and developing
rapidly. As discussed below several animals have already been produced in which
genes of interest in nociceptive pathways have been coupled to GFP. Through
collaborations we have been able to obtain some of these mouse strains and are
in the process of acquiring others. Currently we are breeding a very
interesting mouse in which most of the primary afferents innervating the
epidermis express GFP. Most of this application concerns this mouse. In
addition we will be establishing a colony of mice in which GABAergic inhibitory
neurons are labeled with GFP and some of the studies this will be used for are
also discussed. Several other developments are also discussed to indicate the
overall direction in which this research is developing.
b. Background.
i. Promoters driving production of
marker proteins.
With the sequencing of the
human genome and subsequently that of the mouse it has been possible to
identify the regulatory regions controlling the expression of many genes.
Typically this is a sequence immediately preceding the first translated region
of the gene (exon1). These promoter regions can be used to drive other genes
including those for the fluorescent protein, green fluorescent protein (GFP).
If a DNA sequence consisting of a promoter and GFP is inserted into the genome
it will have in addition to its own copy of this promoter driving its normal
gene, the additional transgene. Now when the normal promoter is activated to
produce its gene product, the transgene will also be activated to produce GFP.
For example, we have recently started investigating a mouse in which a promoter
called Thy 1.2 drives the production of GFP. In this mouse a group of primary
afferents innervating the epidermis is bright green (Figure 1). These fibres
terminate in LII of the spinal cord. Findings on a similar mouse in which a
G-protein couple receptor drives the production of GFP, which also has labeled
epidermal fibres, were reported in January (Zylka et al 2005).
ii. Viral delivery of promoters
driving production of marker proteins.
An alternative route to introducing
the transgene is to use a virus vector such as the adeno-associated virus
(AAV). The viral gene is altered so that it now contains a promoter driving
production of GFP. Now cells taking up the virus and activating this promoter
will turn green. This has been very successfully applied to neurons expressing
melanin concentrating hormone (MCH) in the hypothalamus (van den Pol et al
2004). The MCH neurons taking up the virus turned green permitting the
researchers to selectively target these neurons with patch recording
electrodes. As only the MCH neurons turn green this greatly facilitates
recording from specific neurone types that occur in low density.
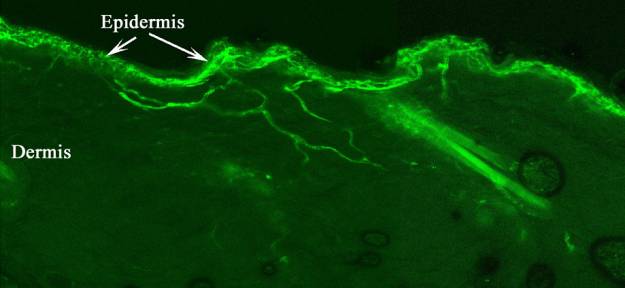
Figure 1. Section through the skin of
transgenic mouse expressing GFP in epidermal fibres (Fresh frozen section no
staining)
iii. Reporter mice and cre-loxP
technology.
Another
development in these methods is to use a system known as the cre-loxP system.
LoxP sequences are short DNA sequences. Typically two or more of these are
introduced into a gene and the gene is said to be “floxed”. Cre-recombinase is
an enzyme from bacteriophages that cross links two loxP sequence and excises
one loxP sequence and the intervening DNA. This has been used to inactivate or
“knockout” genes. However, another interesting use is to label cells. Mice have
been produced that are called reporter mice. These have the sequence promoter-loxP-stop-loxP-reporter
gene. The reporter gene is commonly a gene for a fluorescent protein such
as GFP. In this mouse the gene is not expressed due to the stop
sequence. However, when cre-recombinase is introduced cells in which this is
produced turn green. As discussed above, for promoters driving GFP, the
cre-recombinase can either be produced genetically or via a viral vector. For
example, if a mouse is produced in which a homeobox (Hox) gene drives
the production of cre-recombinase, and this is then crossed with a reporter
mouse in which the neuronal specific promoter tau is linked to GFP, then
all the neurons activating tau, which also activate the hox gene,
will turn green. This technology is rapidly identifying neuronal lineages and
has for example revealed how the ventral floor plate of the spinal cord
differentiates to produce different groups of neurones. The hox genes
regulating the dorsal plate differentiation into dorsal horn neurones are now
being identified. This technology will permit different groups to dorsal horn
neurones to tracked through development and targeted with whole-cell patch
recording electrodes.
iv. Conditional gene knockout using
cre-loxP technology.
The implications of the cre-loxP
system do not stop at simply labeling neurones. If a mouse is generated in which
a specific promoter drives the production of cre-recombinase then when this is
crossed with animals containing “floxed” genes the gene will be inactivated.
Recently, for example a mouse has been produced in which the promoter for a
sodium channel (NaV 1.8) that is unique to small dorsal root
ganglion (DRG) neurons drives the production of cre-recombinase (Stirling et al
2005). Crossing this animal with one in which a gene has been “floxed” will now
inactivate this gene in just the neurons activating NaV1.8. So we
now have a method for inactivating genes in just one group of neurons. An
alternative approach is to introduce the cre-recombinase via a viral vector.
This has been used to examine the role of the NR1 subunit of the NMDA receptor
in nociception. A mouse was produced in which the NR1 gene was “floxed” and
adeno-associated virus expressing the cre-recombinase gene under the regulation
of the human cyclomegalavirus promoter (HCMV) was injected in the cord. This
inactivated NR1 subunit synthesis in the transfected neurons and led to changes
in the development of the second phase response in the formalin test (South et
al 2003). This approach is very valuable in testing new targets for analgesics
when no specific antagonists have been developed.
v. Switching on neurotoxic genes.
In
the same way as discussed for reporter mice it is possible to selectively
lesion neurons. If a mouse with the following sequence
promoter-loxP-Stop-loxP-neurotoxin gene is crossed with an animal in which cre-recombinase
is produced by a neuron specific promoter, such as the NaV1.8
promoter, just those neurons expressing this gene would die. This provides a
method for selectively lesioning component neurons in pain pathways to see what
changes occur. For example applying this to the neurokinin 1 (NK1)
receptor expressing neurons would be a much cleaner method of lesioning these
neurons than the existing method with saporin conjugated substance P.
vi. Summary.
Essentially neurobiology is entering a
new era in which new genomic methods open huge possibilities. In theory we
could have a colour coded nervous system, lesion any selected neuronal group,
separate any neurone type for gene expression analysis and track any neurone
type through development, in culture or as it responds to insults such as nerve
injury.
vii. Evaluation and exploitation of
these technologies.
In
view of this potential we have been rapidly reconfiguring our laboratories to
exploit these methods. We have assembled several electrophysiology systems
equipped for whole-cell patch electrode studies from fluorescently labeled
neurons in tissue slices and dissociated cultures. Several strains of mice have
been imported in which different populations of neurons fluoresce green. Several
reporter mice lines have been established. One mouse line expressing
cre-recombinase has been imported and contacts have been established with
several other groups breeding these mice. In collaboration with Professor John
Quinn several viral vectors are being constructed including ones in which
galanine and the NK1 promoter regions drive production of GFP. We
are in position immediately to investigate the functions of epidermal primary
afferents and a number of specific experiments to be conducted with these
animals are discussed. We also have on order a mouse in which inhibitory
interneurones containing GABA are labelled.
Other work at an earlier stage of development is also discussed.
We
have been given a remarkable mouse in which all the small DRG neurons that bind
IB4 lectin express GFP. There is no expression in calcitonin gene related
peptide (CGRP) or substance P containing primary afferents. All the GFP labeled
afferents innervate hairy skin and reveal fine details of this innervation
(Figure 1). Most cross the dermis without branching and enter the epidermis
where they break up into huge numbers of fine terminal branches. The central
terminals of these primary afferents innervate LII, the substantia gelatinosa
(SG). Hence, we now know that one of the primary functions of the SG is
analysis of epidermal information. Many of these IB4 binding afferents express
TRPV1 channels that are activated by noxious heat, acid and capsaicin.
Activation of these afferents would produce the sensation of burning pain,
which can be so debilitating in some neuropathic pain patients. We propose that this system of primary
afferents transducer signals that have to be detected in the epidermis. Thus
they would be concerned with noxious and non-noxious thermal reception, itch
and probably pleasurable touch. We would hypothesise that different groups of
these afferents would express different TRP channels and different Mgr-family
G-protein coupled receptors. This will be tested with by whole-cell patch
recording from the dissociated DRG. The
expression of GFP will permit direct targeting of electrodes on these specific
cells. Additionally this opens up a range of other possibilities including
studies of : the effects of peripheral applications of substances such as
capsaicin (they could also be used to evaluate any topically applied compound),
the effects of peripheral nerve damage, dorsal root damage and dorsal root
regeneration. These cells can also be separated in a fluorescent cell sorter
for gene expression studies (not part of the current proposal due to expense).
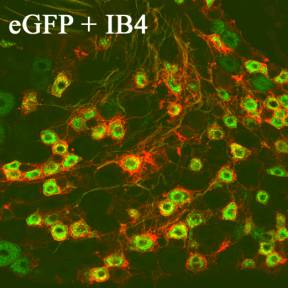
Figure 2. Section of DRG from Thy
1.2-gfp mouse. Stained with biotinylated IB4-lectin and strepatavidin Cy3 (red)
showing colocalisation of IB4 and GFP (Note the GFP can be seen in intact
ganglia with no staining).
2. Gad-GFP and VACh-cre mice.
One of the key problems in working
out dorsal horn circuity is identification of neurone types. Recently the first
studies exploiting GFP to identify spinal interneurones were published (Hantman
et al 2004, Heinke et al 2004). Heinke et al (2004) used a commercially
available (Jaxmice) mouse in which the promoter for enzyme glutamic acid
decarboxylase (GAD) drives GFP production and hence, marks inhibitory neurons
producing GABA. We are in the process of importing breeding pairs of these mice
from Jackson Labs to establish a colony. Apart from their use to record from
inhibitory interneurones in LI-LIII of the dorsal horn, they offer a simple way
of evaluatating GABA neurone loss in neuropathic models. A collaborator (Dr
Misawa) is also sending us mice in which the promoter for the vesicular
transporter for acetylcholine (VACh) drives the production of cre-recombinase
(Misawa H et al 2003). We will cross this animal with our reporter mice with
the intention of producing green LIII cholinergic neurons. These are critically
involved in inhibitory processes in the dorsal horn, but because of their low
density it has not been possible to study them. If this works it will solve
this problem.
3. AAV NK1-GFP,
Galanine-GFP, NK1-cre.
AAV
are being made in Professor Quinn’s laboratory. These will be injected into the
lumbar cords of neonatal rats (NK1-GFP, Galanine-GFP) or in adult reporter mice
(NK1-cre). This is with the intention of producing green NK1
or galanine neurons in the lumbar spinal cord. If this is successful we will be
able to target these for electrophysiological studies.
Methods.
Transgenic mouse breeding and
screening. Virus production. Standard
RT-PCR methods will be used to identify mice carrying the genes of interest in
our breeding programs. Viruses expressing promoters linked to GFP or
cre-recombinase have been produced or are being produced by Professor John
Quinn as part of an ongoing collaboration.
Electrophsiology. Both the applicants have a strong
background in electrophysiology. Dr Morris has developed and employed spinal
cord recording methods for nearly twenty years (eg Cheunsuang et al 2002) and
has extensively investigated cultured DRG (eg. Thippeswamy et al 2004). Dr
Barrett-Jolley, has also an extensive background in patch-clamp work,
particularly from the hypothalamus, but also brings to the project valuable
experience in evaluating the biophysical properties of dissociated cells.
Recordings will be made from neurones identified by their expression of GFP. In
the thy1.2-gfp mouse recordings will be made from dissociated DRG
neurons maintained in short term culture. Their biophysical properties and
responses to a range of agonists will be examined. Although some studies have
produced data for DRG neurons shown subsequently to bind IB4 lectin in the
present studies we will have the advantage of being able to selectively target
these neurons. Their expression of TRP channels, ASIC channels and H1 histamine
receptors will be evaluated by local application of thermal stimuli, capsaicin,
menthol, acid at different pH and histamine. The neurones will be tested under
voltage clamp conditions and their current-voltage relations analysed. Some of
the ligands for the recently identified Mrg family of G-protein coupled
receptors will also be tested. In spinal cord slices preliminary recordings
will also be made from GAD expressing neurons. Depending on the success of
viral transfection and breeding similar studies will be undertaken of Ach, NK1
and Galanine neurons. These will act as proof of method pilot studies for
further grant applications to the Wellcome trust and BBSRC.
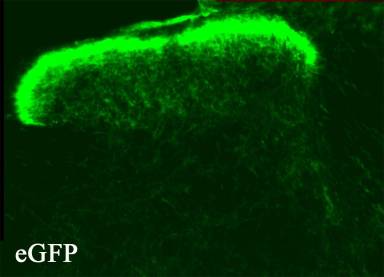
Figure 3. Transverse section of the
dorsal horn of the thy 1.2-gfp mouse to show distribution of GFP. Fresh
frozen wet mounted section, no staining, the green is, as it would be observed
in a living slice for recording.
Dorsal rhizotomy, sprouting, growth
factors etc. It is immediately
apparent that the Thy1.2-gfp mouse could also permit tracking of this
fibre population following dorsal rhizotomy. Here we would get a definite loss
of fibres in the spinal cord the territory denervated would be immediately
apparent by the loss of fluorescence. It is possible that adjacent roots form
sprouts into the denervated region and this could be examined. Some groups have
also claimed that substantial regeneration of a sectioned dorsal root can be
achieved by application of growth factors. However, this has been questioned by
others. The Thy1.2-gfp mouse could solve this issue by having an inbuilt tracer
which would permit detailed observations of fibres regeneration and immediately
show any spared fibres.
Anticipated result
Firstly the experience we obtain in
exploiting these new methods will constitute a major step forward. It is clear,
that even if the specific examples given do not generate new data, the overall
approach will. It is anticipated that the whole-cell patch studies of the
Thy1.2-gfp DRG neurons will reveal a range of transduction properties. We
already know that some express TRPV1 receptors activated by noxious heat,
capsaicin and protons. Some will also express other thermal receptors. However,
the majority are anticipated to express Mrg G-protein coupled receptors which
we will test by applying FMRFamide peptides (Dong et al 2001). Activation of
these receptors is thought to be noxious and these neurons would be an ideal
test situation for antagonists.
The whole cell patch studies on GAD
neurons will permit us to label these cells by intracellular injection and
explore their axon trajectories and contacts. We anticipate that this will
reveal several neurone populations. These studies will be used to apply for
more extensive funding. Similarly the other studies targeted at labeling
specific populations will be used as proof of principle studies.
The nerve injury studies are
anticipated to result in a region of LII loosing its fluorescent labeling. As a
result we will be able to target this region specifically. From earlier studies
on complete sciatic nerve transection we anticipate that no evidence of Ab-fibre sprouting will be obtained.
However, we do expect to see abnormal inhibitory processes with some neurons
being strongly inhibited, and others loosing inhibition. The organization of
these changes may contribute strongly to our understanding of neuropathic pain
processes.
In the epidermis it is anticipated
that application of capsaicin will cause many fine epidermal fibre terminals to
shrink back from the epidermis as shown by others. However, we would anticipate
that some fibres that do not express TRPV1 ion channels would not degenerate.
Depending on the outcome of these experiments further studies of the functions
of the remaining fibres would be possible. Conversely, if all the fibres do
shrink back it presents an interesting question as to how capsaicin is acting.
The dorsal rhizotomy studies should
reveal that GFP labeled afferents regenerate to the junction along the dorsal
root where myelination by Schwann cells changes to myelination by
oligodendrocytes. This junction is readily seen by staining for astrocytes. In
the spinal cord a gap will be seen with no gfp in LII. We anticipate that no
fibres will enter the dorsal root but that some branching will occur in the
spinal cord form adjacent roots.
Relevant
publications
Cheuansuang O and Morris R
(2000) Spinal lamina I neurones which express neurokinin 1 receptors: 1.
Morphological analysis, Neuroscience 97 :335-345
Nazli M., Hismiogullari E.S.,
Thippeswamy T and Morris R., (2001) How central is nitric oxide (NO) to
the activation of c-fos in spinal neurones following noxious peripheral
stimulation in the rat ? Brain Res 888 :172-157
Thippeswamy T. and Morris
R., (2001) Evidence that nitric oxide induced sythesis of cGMP only occurs
in a paracrine and not autocrine fashion and that the site of its release can
be regulated: studies in dorsal root ganglia in vivo and in vitro.
Nitric Oxide 5 : 105 – 115
Thippeswamy. T. and Morris R.,
(2001) Inhibition of nitric oxide synthesis in axotomised primary sensory
neurones causes cell death : Evidence for a neuroprotective role for nitric
oxide in injured neurones in vivo. Neuroscience Research 40 : 37 –44
Thippeswamy. T. McKay J.S. and Morris R. (2001) Bax and caspases are
inhibited by endogenous nitric oxide in dorsal root ganglion neurones in
vitro. European J. Neuroscience
14:1229-1236
Cheuansuang O., Maxwell D.
and Morris R., (2002) Spinal lamina I neurones which express neurokinin
1 receptors: II. Electrophysiological characteristics, responses to primary afferent stimulation
and effects of a selective μ-opioid
receptor agonist. Neuroscience. 111 :423-434
Stewart A.L, Morris R,
Bannatyne B.A., Gordon S.L.G., Singleton A.G. and Maxwell D.J. (2003).
Preliminary evaluation of methods for the study of identified excitatory and
inhibitory neurones in LI-LIII of the rat spinal dorsal horn. British
Neurosci. Assoc. Abst 17: 34.03
Morris
R, Cheunsuang O, Stewart
A.L. and Maxwell.D., (2004) Spinal dorsal horn neurone targets for nociceptive
primary afferents: do single neurone morphological characteristics suggest how
nociceptive information is processed at the spinal level. Brain Research Review
46 :173 –190
Morris R, Arber S, Kramer I, Sigrist M,
Belle M and Cheunsuang O.,
(2004) Evaluation of the distribution of labeling in primary afferents in a
Thy-1.2 promoter-enhanced green fluorescent protein (GFP) transgenic mouse:
evidence that the substantia gelatinosa is primarily concerned with sensory information
transduced in the epidermis. Presented
at the 2nd James Black Symposium, Cambridge.
Thippeswamy
T, Jennifer S. McKay J.S, Morris R, Quinn J, Wong L-F and Murphy D.,
(2004) Glial-mediated neuroprotection: evidence for the protective role of the
NO-cGMP pathway in the peripheral nervous system via neuron-glial
communication. Glia 154 : 153-164
Thippeswamy T McKay J.S. Quinn J.P and Morris R., (2005) Either Nitric Oxide or Nerve Growth
Factor is required for dorsal root ganglion neurons to survive during embryonic
and neonatal development. Develop. Brain Res. 154 : 153-164
Abu Zaki & Barrett-Jolley, R.,
(2002). Rapid neuromodulation by cortisol in the rat paraventricular nucleus:
An in vitro study. British
Journal of Pharmacology 137:87-97.
Barrett-Jolley,
R. (2001). Nipecotic
acid directly activates GABA(A)-like ion channels. British Journal of Pharmacology
133:673-678.
Barrett-Jolley,
R, Pyner, S. & Coote,
J.H. (2000). Measurement of Voltage-Gated Potassium Currents in Identified
Spinally-Projecting Sympathetic Neurones of the Paraventricular Nucleus. Journal
Neuroscience Methods. 102
:25-33.
Other
relevant publications.
Dong
X, Han S-K, Zylka M.J., Simon M.L. and Anderson D.J., (2001) A diverse family of
GPCRs expressed in specific subset of nociceptive neurons. Cell 106 : 619-632.
Hantman AW,
van den Pol AN, Perl ER. (2004)Morphological
and physiological features of a set of spinal substantia gelatinosa neurons
defined by green fluorescent protein expression. J Neurosci. 24 : 836-42.
Heinke
B, Rischeweyh R., Fosthuber L., Wunderbaldinger G, and Sandkuhler J. (2004) Neurochemical and morphological properties
of a subgroup of GABAergic spinal lamina II neurones identified by expression
of green fluorescent protein in mice. J. Physiol 560 :249-266
Misawa
H., Nakata K., Toda K., Matsuura J., Oda Y, Inoue H, Tatneno M. and Takahashi
R., (2003) VAChT-Cre, fast and VAChT-cre slow: postanatal expression of cre
recombinase in somatomotor neurones with different onset. Genesis 37 :44-50.
van den Pol
AN, Acuna-Goycolea C, Clark KR, Ghosh PK. (2004) Physiological properties of hypothalamic
MCH neurons identified with selective expression of reporter gene after
recombinant virus infection. Neuron 42: 635-652
South
S.M., et al (2003) A conditional deletion of the NR1 subunit of the NMDA
receptor in adult spinal cord dorsal horn reduces NMDA currents and
injury-induced pain. J Neuroscience 23 :5040-5031.
Stirling
L.C., Forlani G., Baker M.D., Wood J.N., Matthews E.A., Dickenson A.H. and
Nassar M.A., (2005) Nociceptive-specific gene deletion using heterozygous NaV
1.8-Cre recombinant mice. Pain 113 :27-36.
Zylka
M.J., Rice F.L., and Anderson D.J. (2005) Topographically distinct epidermal
nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 46
:17-25.
Justification
of costs.
The salary will be used to provide a
stipend for a research student who would be registered for a Ph.D. The rate of
the stipend is based on that provided by the BBSRC for research students. The
student’s fees and further running expenses will be covered from funds in a
Departmental account (see covering letter). The requested consumable costs will
be spent on maintenance of mice, immunochemicals, drugs and other routine
items. The travel money is the same amount as given to Research students by the
BBSRC to cover travel to national and international meetings. The student will
also be encouraged to apply for funds from other sources to attend
International Pain meetings. Student stipends and their running costs are not
subject to overhead costs by the University of Liverpool.
There is a great shortage of trained research students entering Pain research and in particular into functional studies using electrophysiological methods. This is at a time when methods are emerging which will greatly advance this field. Apart from the importance of the work to be conducted this grant would have the added advantage of training a scientist at an early stage of their career who it is hoped would continue to contribute to this field after obtaining their doctorate.