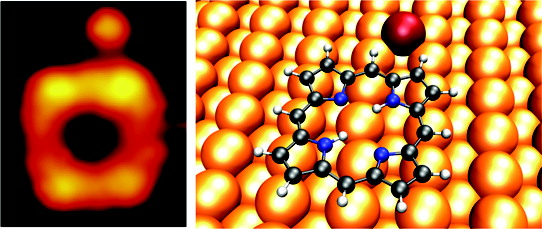

We investigate the adsorption and conformation of free-base porphines on Cu(110) using STM, reflection absorption infrared spectroscopy, and periodic DFT calculations in order to understand how the central polypyrrole macrocycle, common to all porphyrins, interacts with a reactive metal surface. We find that the macrocycle forms a chemisorption bond with the surface, arising from electron donation into down-shifted and nearly degenerate unoccupied porphine π-orbitals accompanied with electron back-donation from molecular π-orbitals. Our calculations show that van der Waals interactions give rise to an overall increase in the adsorption energy but only minor changes in the adsorption geometry and electronic structure. In addition, we observe copper adatoms being weakly attracted to adsorbed porphines at specific molecular sites. These results provide important insights into porphyrin-surface interactions that, ultimately, will govern the design of robust surface-mounted molecular devices based on this important class of molecules.
The American Chemical Society holds the copyright for the published article. More information can be found on their website.
Limitted copies of the full text are available through the ACS Articles on Request service by following this link.
1The Surface Science Research Center, The University of Liverpool, Liverpool L69 3BX, U.K.
2 Department of Applied Physics, Chalmers University of Technology, SE-412 96 Göteborg, Sweden
3 London Centre for Nanotechnology and Department of Chemistry, University College London, London WC1E 6BT, U.K.