Chemistry of Materials, 22 (2010) 6598–6615

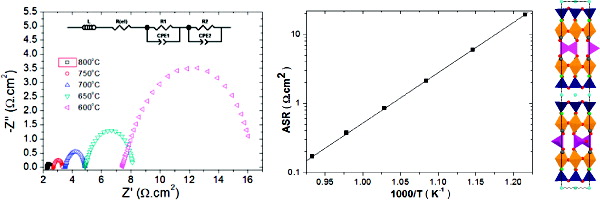
Ba1.6Ca2.3Y1.1Fe5O13 is an Fe3+ oxide adopting a complex perovskite superstructure, which is an ordered intergrowth between the Ca2Fe2O5 and YBa2Fe3O8 structures featuring octahedral, square pyramidal, and tetrahedral B sites and three distinct A site environments. The distribution of A site cations was evaluated by combined neutron and X-ray powder diffraction. Consistent with the Fe3+ charge state, the material is an antiferromagnetic insulator with a Néel temperature of 480-485 °C and has a relatively low d.c. conductivity of 2.06 Scm-1 at 700 °C. The observed area specific resistance in symmetrical cell cathodes with the samarium-doped ceria electrolyte is 0.87 Ωcm2 at 700 °C, consistent with the square pyramidal Fe3+ layer favoring oxide ion formation and mobility in the oxygen reduction reaction. Density functional theory calculations reveal factors favoring the observed cation ordering and its influence on the electronic structure, in particular the frontier occupied and unoccupied electronic states.
The American Chemical Society holds the copyright for the published article. More information can be found on their website.
1Department of Chemistry, University of Liverpool, Liverpool, UK
2Department of Physics, University of Liverpool, Liverpool, UK
3ISIS Facility, Rutherford Appleton Laboratory, Chilton, UK