Research
Protonic Conductors
Research
Protonic Conductors
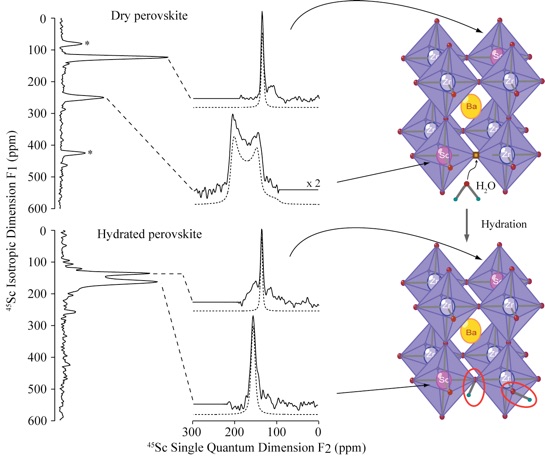
One of the ways to lower the high operating temperatures of Solid Oxide Fuel Cells (SOFCs) is to replace oxygen-ion conducting electrolytes by protonic conductors. Indeed, many of the anionic conductors used in SOFCs are also potential protonic conductors after hydration, perovskite-type materials showing the best results at intermediate temperatures. The oxygen vacancies, which are responsible for the anionic conduction at high temperatures, also allow for hydration of the structure: one molecule of water can react with an oxygen vacancy and an oxide ion to form two OH groups, generally referred to as the protonic defects. The proton ions of these defects can hop from oxygen ion to oxygen ion at intermediate temperatures giving rise to protonic conduction. An example of such process is given below in the case of the hydration of Sc doped BaZrO3 perovskite.
Such hydration process could be follow by NMR spectroscopy at high field by recording ex-situ 45Sc NMR two-dimensional Multiple Quantum Magic Angle Spinning (MQMAS) experiments of the perovskites before and after hydration. Analysis of both peak positions and lineshapes of the NMR data allow for the observation of the disappearance of 5 fold coordinated Sc and appearance of 6 fold coordinated Sc bearing a protonic defect ScOH in agreement with Sc atom being neaby an oxygen vacancy.
see for example:
Proton trapping in ytttrim-doped barium zirconate. Y. Yamazaki, F. Blanc, Y. Okuyama, L. Buannic, J. C. Lucio-Vega, C. P. Grey, S. M. Haile, Nat. Mat., 2013, 12, 647-651.
Probing cation and vacancy ordering in the dry and hydrated yttrium substituted BaSnO3 perovskite by NMR spectroscopy and first principles calculations: implications for proton mobility. L. Buannic, F. Blanc, D. S. Middlemiss, C. P. Grey, J. Am. Chem. Soc., 2012, 134, 14483-14498.
Probing the local structures and protonic conduction pathways in scandium substituted BaZrO3 by multinuclear solid-state NMR spectroscopy. L. Buannic, F. Blanc, I. Hung, Z. Gan, C. P. Grey, J. Mat. Chem. 2010, 20, 6322-6332.
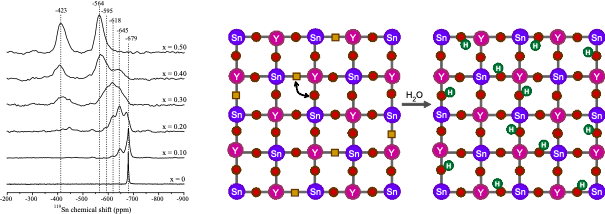
Hydrated BaSn1-xYxO3-x/2 is a protonic conductor that, unlike many other related perovskites, shows high conductivity even at high substitution levels. A joint multinuclear NMR spectroscopy and density functional theory (total energy and GIPAW NMR calculations) investigation of BaSn1-xYxO3-x/2 (0.10 < x < 0.50) was performed to investigate cation ordering and the location of the oxygen vacancies in the dry material. The DFT energetics show that Y doping on the Sn site is favored over doping on the Ba site. The 119Sn chemical shifts are sensitive to the number of neighboring Sn and Y cations, an experimental observation that is supported by the GIPAW calculations and that allows clustering to be monitored: Y substitution on the Sn sublattice is close to random up to x = 0.20, while at higher substitution levels, Y-O-Y linkages are avoided, leading, at x = 0.50, to strict Y-O-Sn alternation of B-site cations.
These results are confirmed by the absence of a “Y-O-Y” 17O resonance and supported by the 17O NMR shift calculations. Although resonances due to six-coordinate Y cations were observed by 89Y NMR, the agreement between the experimental and calculated shifts was poor. Five-coordinate Sn and Y sites (i.e., sites next to the vacancy) were observed by 119Sn and 89Y NMR, respectively, these sites disappearing on hydration. More five- coordinated Sn than five-coordinated Y sites are seen, even at x = 0.50, which is ascribed to the presence of residual Sn-O-Sn defects in the cation-ordered material and their ability to accommodate O vacancies.
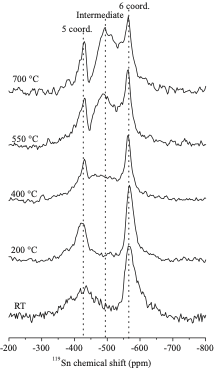
High-temperature 119Sn NMR reveals that the O ions are mobile above 400 °C, oxygen mobility being required to hydrate these materials. The high protonic mobility, even in the high Y-content materials, is ascribed to the Y-O-Sn cation ordering, which prevents proton trapping on the more basic Y-O-Y sites.