Research
Fast Lithium Ion Conductors
Research
Fast Lithium Ion Conductors
Replacing liquid electrolytes with solid ceramics or polymers in next-generation battery technologies is of growing interest due to the major impact that will result from the increase in lifetime and safety, higher power outputs and higher energy densities expected in energy storage appliances. Lithium has become the element of choice in most battery designs because of the high mobilities arising from its small ionic radius. Hence, a major goal of the field is to discover lithium-based ceramics with ionic conductivities that equal or surpass current liquid electrolytes and investigate their diffusion mechanisms. We are exploiting the ability of NMR to probe dynamics to investigate materials with potential fast Li ion conductivity properties. We are specifically targeting a range of newly synthesize solid state Li ion materials, in collaboration with Prof. Rosseinsky at the University of Liverpool and Prof. West at the University of Sheffield.
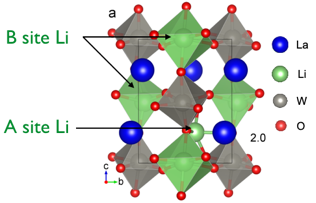
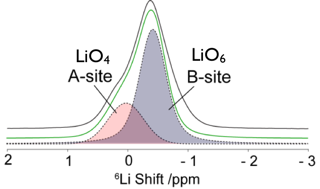
La3Li3W2O12 is a new class of ABO3 perovskite with Li ions on both the A and B sites and adopts a monoclinic double perovskite structure (La1.5Li0.5)WLiO6 with rock salt order of W and Li ions on the B site. 6Li Magic Angle Spinning NMR spectrum reveals the presence of two Li sites containing a lower coordinated Li ion (on the A site) in addition to the B-site LiO6 site, experimentally verifying the Density Functional Theory calculations. Li ion dynamics probed by a range of approaches including variable temperature 6Li and 7Li NMR line shape analysis and relaxation measurements revealed a Li diffusion pathway with low activation energy comparable with the best oxide Li ion conductors. Ab initio molecular dynamics establishes that the Li ion dynamics occur by a pathway involving a series of multiple localized Li hops between two neighboring A-sites with additional possible pathways involving Li exchange between the A- and B-sites.
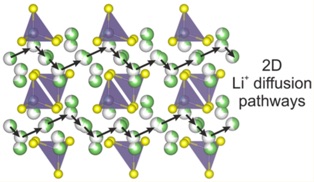
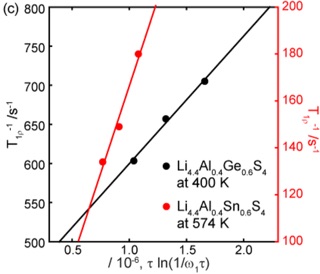
Many of the room temperature fastest solid state Li ion conductors reported are based non-oxide sulfide containing materials due to the greater ionic size and polarizability of the sulfide anion. We identified the new crystalline phase Li4.4Al0.4Ge0.6S4, and investigated the structure and Li ion dynamics of the family of structurally related materials Li4.4M0.4M′0.6S4 (M = Al3+, Ga3+ and M′ = Ge4+, Sn4+) using a combine approach involving neutron and X-ray diffraction techniques, conductivity, NMR and computational studies. The Al- homologues adopt a layered close-packed structure with a new arrangement of tetrahedral (M/M′) sites and a novel combination of ordered and disordered lithium vacancies. A combination of variable temperature 7Li solid state NMR and ab initio molecular dynamics calculations on selected phases showed that long-range lithium transport diffusion occurs with a low energy barrier of 0.17 eV. In particular, the frequency dependence of the NMR relaxometry data reveals a two-dimensional diffusion process.
see for example:
Computational Identification and Experimental Realisation of Lithium Vacancy Introduction into the Olivine LiMgPO4, L. Enciso-Maldonado, M. S. Dyer, M. D. Jones, M. Li, J. L. Payne, M. J. Pitcher, M. K. Omir, J. B. Claridge, F. Blanc, M. J. Rosseinsky, Chem. Mater. 2015, 27, 2074-2091
Ion Dynamics in Li2CO3 Studied by Solid-State NMR and First-Principles Calculations, M. T. Dunstan, J. M. Griffin, F. Blanc, M. Leskes, C. P. Grey, J. Phys. Chem. C. 2015, 119, 24255-24264
La3Li3W2O12: Ionic Diffusion in a Perovskite with Lithium on Both A- and B-Sites, A. B. Santibáñez-Mendieta, C. Didier, K. K. Inglis, A. J. Corkett, M. J. Pitcher, M. Zanella, J. F. Shin, L. M. Daniels, A. Rakhmatullin, M. Li, M. S. Dyer, J. B. Claridge, F. Blanc*, M. J. Rosseinsky*, Chem. Mater. 2016, 28, 7833-7851
Lithium Transport in Li4.4M0.4M’0.6S4 (M= Al, Ga and M’= Ge, Sn): Combined Crystallographic, Conductivity, Solid State NMR and Computational Studies, B. T. Leube, K. K. Inglis, E. Carrington, P. Sharp, J. F. Shin, A. R. Neale, T. D. Manning, M. J. Pitcher, L. J. Hardwick, M. S. Dyer, F. Blanc, J. B. Claridge, M. J. Rosseinsky, Chem. Mater. 2018, 30, 7183-7200
Synthesis and Characterisation of the New Oxyfluoride Li+ Ion Conductor, Li5SiO4F, B. Dong, J. Yan, B. Walkley, K. K. Inglis, F. Blanc*, S. Hull, A. R. West*, Solid State Ionics, 2018, 327, 64-70