




|
Research |

|
Reversible phosphorylation of key structural and regulatory proteins, from histones to the transcriptional machinery, is acknowledged to be an important mechanism of regulating chromosome architecture, |
|
Research
|
|
Chromosome biology & epigenetics |
|
Chromosome biology & epigenetics |
|
Home Research People Publications News Contact us |
|
gene expression and epigenetic programming. Aberrant (de)phosphorylation of chromosome-associated proteins has recently been implicated in the development of number diseases, including cancer. Therefore, understanding the regulation of the relevant protein kinases and phosphatases is of increasing importance.
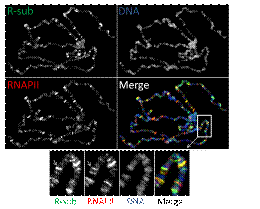
In Drosophila, PP1 is localised to many sites on chromosomes (for an e.g. of a stained polytene spread see above), most likely through interaction with a variety of different regulatory subunits that modify PP1’s activity towards specific substrates. Our goal is to understand the role and regulation of these PP1-containing chromosome-associated complexes. In particular, we aim to i) determine where precisely on chromosomes PP1 is located; ii) establish which regulatory subunit targets PP1 to each of these loci; iii) analyse the effect of each holoenzyme on transcription and chromatin modification at specific loci; iv) identify the substrates of each holoenzyme; v) determine the effects of (de)phosphorylation (e.g. during development or in our Drosophila cancer models).
Our focus is on a number of key chromosome-associated PP1-binding proteins that we have identified (Bennett 2005; Bennett et al 2006). One such protein is Trithorax (Trx), a histone methyltransferase that is involved in maintaining active transcription of homeotic genes and other genes after their initial activation. PP1 binds directly to Trx protein, co-localizes with Trx on polytene chromosomes and antagonizes trx function in vivo (Rudenko et al. 2003, 2004). The human homologue of Trx, ALL-1/HRX/MLL, is affected by over 30 different chromosomal fusions that lead to leukaemia, highlighting the importance that transcriptional control by Trx genes has to human health.
|
|
This work is funded by: |

|
Cell signalling and Development in Drosophila |
|
Bennett Lab |
|
Changes in chromosome architecture and the distribution of epigenetic modifications and chromatin-associated proteins can be easily monitored in Drosophila on polytene chromosomes, which have long provided a model for examining events that occur during interphase. |