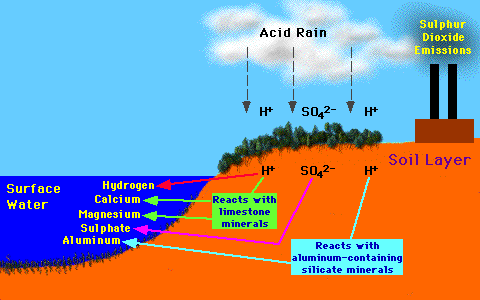
Lake acidification begins with the deposition of the by-products acid
precipitation (SO4 and H ions) in terrestrial areas
located adjacent to the water body. Hydrologic processes then move these
chemicals through soil and bedrock where they can react with limestone and
aluminum-containing silicate minerals. After these chemical reactions, the
leachate continues to travel until it reaches the lake. The acidity
of the leachate entering lake is controlled by the chemical composition
of the effected lakes's surrounding soil and bedrock. If the soil and bedrock
is rich in limestone the acidity of the infiltrate can be reduced by the
buffering action of calcium and magnesium compounds. Toxic aluminum (and
some other toxic heavy metals) can leach into the lake if the soil and bedrock
is rich in aluminum-rich silicate minerals. This can reach concentrations
sufficient to be toxic to fish.
