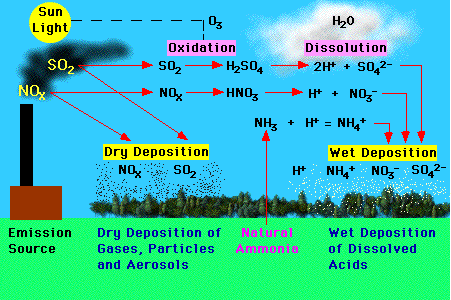
Several processes can result in the formation of acid deposition. Nitrogen oxides (NOx) and sulfur dioxide (SO2) released into the atmosphere from a variety of sources call fall to the ground simply as dry deposition. This dry deposition can then be converted into acids when these deposited chemicals meet water. Most wet acid deposition forms when nitrogen oxides (NOx) and sulfur dioxide (SO2) are converted to nitric acid (HNO3) and sulfuric acid (H2SO4) through oxidation and dissolution. Wet deposition can also form when ammonia gas (NH3) from natural sources is converted into ammonium (NH4).
